
Prognostic analysis of 152 patients with distant metastasis after intensity-modulated radiotherapy for nasopharyngeal carcinoma
Introduction
Nasopharyngeal carcinoma (NPC) is one of the most common malignancies of the head and neck. It is unevenly distributed all over the world, with especially high prevalence in Southeast Asia and southern China (1). Radiotherapy is the primary treatment method for NPC due to its anatomical and pathological characteristics. Anatomically, the nasopharynx is deep inside with complex surrounding organs and tissues, thus making it difficult to surgically remove the tumors completely. Pathologically, the most common type of NPC is poorly differentiated squamous cell carcinoma, which is highly sensitive to radiation. In conventional two-dimensional radiotherapy, local recurrence and distant metastasis are the main failure modes for locally advanced NPC (2,3). With the development of three-dimensional conformal intensity-modulated radiotherapy (IMRT), the local control rate of locally advanced NPC has significantly improved, with a 3-year local recurrence-free survival of >90%. Distant metastasis is becoming the main problem affecting patients’ survival after IMRT treatment (4,5).
The prognosis of metastatic NPC is poor, with a short median survival time (6,7). There is no standard treatment plan for this condition. Currently, chemotherapy-based comprehensive treatments are commonly used for metastatic NPC patients while considering various factors, such as the general condition of the patient, age, timing of metastasis, metastatic site, number of involved organs, and number of metastases, etc. (4,8-10).
In recent years, with the development of cancer molecular biology, numerous molecular targeted anti-tumor drugs have been approved in the United States and Europe. Due to their high specificity, targeted drugs could reduce the side effects of conventional chemotherapy and radiotherapy and provide better tolerance for patients with metastatic NPC. However, targeted therapy is still under investigation, and the long-term efficacy and safety of targeted anti-tumor drugs needs to be further validated (11-14).
Local palliative therapy is another important option for the treatment of metastatic NPC. Some patients could reach long-term survival or even clinical cure upon receiving such palliative treatments. However, there is currently no standardized treatment strategy. Most results mentioned above were achieved with conventional two-dimensional radiotherapy technology, and very few studies have investigated the application of IMRT in distant metastatic NPC.
In the present study, we analyzed the survival characteristics for distant metastatic NPC patients after radical IMRT treatment, with the hope of screening out factors that improve survival, and to help establish individualized treatment strategies with better prognosis. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-21-1279).
Methods
Study design
A retrospective analysis was conducted on 157 NPC patients with distant metastasis after initial IMRT treatment that was admitted to the Department of Radiotherapy of The First Affiliated Hospital of Air Force Medical University from January 2006 to December 2017.
Ethical approval
The present study was approved by the Ethics Committee of the First Affiliated Hospital of Air Force Medical University (ethical approval number: KY20172007-1). All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was taken from all the patients.
Inclusion criteria
The inclusion criteria were as follows: (I) explicit nasopharyngeal histopathology diagnosis before initial treatment; (II) no distant metastasis observed by chest computed tomography (CT), abdominal B-ultrasound, and bone single photon emission computed tomography (SPECT). If distant metastasis occurred after radical radiotherapy, physical examinations, and nasopharyngeal fiberscope examinations, nasopharyngeal + neck magnetic resonance imaging (MRI) scans were conducted to exclude nasopharyngeal recurrence or cervical lymph nodes metastasis. In total, 152 patients were included, with a median follow-up of 43 months.
Radiotherapy
All patients were initially treated with radical IMRT therapy. For CT simulation positioning, the patients were placed in the supine position and fixed using a head-neck-shoulder mold. The scanning ranged from the top of the head to 3 cm below the clavicle, and venography was simultaneously performed, with a layer thickness of 3 mm and a layer spacing of 3 mm. Patient images from the enhanced CT positioning and MRI were merged for target area delineation.
The gross tumor volume (GTV) was divided into two parts: the nasopharyngeal lesion, as shown based on the imaging (GTVnx), and the involved lymph nodes, as determined by the presence of enlarged nodes that met the diagnostic criteria (GTVnd). Clinical target volumes (CTV1) is clinical high-risk target area, which includes the entire nasopharyngeal mucosa, posterior nasal cavity, 1/3 of the posterior maxillary sinus, posterior ethmoid sinus, parapharyngeal space, cranial base openings, inner and outer plates, slopes and anterior 1/3 of the first cervical vertebrae, part of the oropharynx, and the surrounding high-risk lymph node drainage area. CTV2 is low-risk cervical lymphatic drainage area. Each of the above target areas was extended by 3 mm to form respective planning target volumes (PTVs).
The prescription doses were as follows: GTVnx, 66.0–74.25 Gy/30–33 F; GTVnd, 66.0–72.4 Gy/30–33 F; PTV1, 56.0–63.5 Gy/29–33 F; and PTV2, 50.4–53.2 Gy/28 F. The requirements for the PTV doses were as follows: (I) an area <93% of the prescribed dose must be <3% by volume; (II) an area that is >105% of the prescribed dose must be <20% by volume; and (III) an area that is >110% of the prescription dose was not allowed. The vital organ dose-volume limit was determined according to the Radiation Therapy Oncology Group (RTOG) 0225 report: parotid gland: 50% volume ≤30 Gy or an average dose ≤26 Gy; brainstem, optic chiasm, and optic nerve: 54 Gy or 1% volume ≤60 Gy; spinal cord: 45 Gy or 1 cc volume ≤50 Gy; mandible and temporomandibular joint: 70 Gy or 1cc volume ≤75 Gy; and temporal lobe: 60 Gy or 1 cc volume ≤65 Gy.
Chemotherapy
The chemotherapy regimen used for patients in our institution was platinum (cisplatin)-based.
Follow-up
All patients received regular follow-up after initial radiotherapy either by telephone or hospital visits. The Common Terminology Criteria for Adverse Events CTCAE5.0 classification was used to evaluate treatment side effects. Patients were followed up every 3 months in the first year, every 6 months in the second and third years, and annually after 3 years. Patient follow-up involved general physical examinations, electronic nasopharyngoscopy, nasopharynx and neck enhancement CTs or MRIs, chest X-rays or CTs, abdominal B-ultrasound, and whole-body bone ECT scans. Head MRIs were performed when necessary.
Statistical analysis
Statistical analysis was performed using SPSS 17.0 software (SPSS Inc. Released 2008. SPSS Statistics for Windows, Version 17.0. Chicago: SPSS Inc., USA). The Kaplan-Meier method and log-rank tests were used to calculate and compare patient survival rates. P values <0.05 were considered statistically significant.
Results
Clinical and demographic characteristics
Of the 152 patients included in this study, 109 (71.7%) were men, and the median age of all patients at initial diagnosis was 48 (range, 13–81). Based on the American Joint Committee on Cancer classification (7th edition), 17 patients were classified as stages I–II at initial diagnosis, and 135 patients were classified as stages III–IV at initial diagnosis. The general clinical and demographic characteristics of all patients are shown in Table 1.
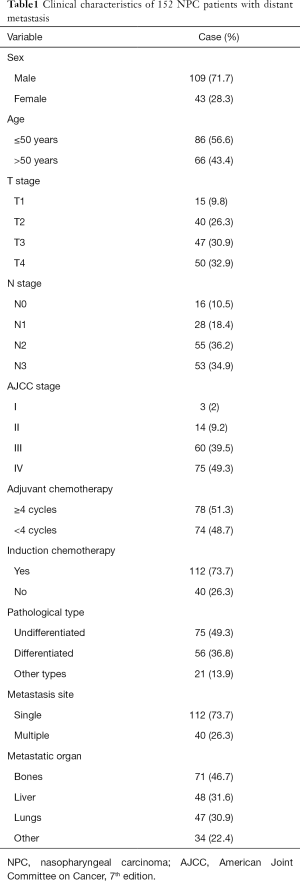
Full table
Chemotherapy
Overall, 136 (89.5%) patients received chemotherapy; 112 (73.7%) received 2–6 cycles of induction chemotherapy, with regimens including cisplatin + fluorouracil (n=28), docetaxel + cisplatin (n=50), docetaxel + cisplatin + fluorouracil (n=10), and gemcitabine + cisplatin (n=24). Also, 130 (85.5%) patients received concurrent chemotherapy with cisplatin for an average of 2–3 cycles. Another 107 (70.4%) patients received 2–6 cycles of adjuvant chemotherapy, including cisplatin + fluorouracil (n=34), docetaxel + cisplatin (n=63), and gemcitabine + cisplatin (n=10).
Follow-up
As of December 2017, 98.7% of patients were retained in care. The median overall follow-up was 43 months. Two patients were lost to follow-up. In total, 128 (84.2%) patients were followed for 3 years, and 101 (66.4%) patients were followed for 5 years.
Efficacy
The median interval time from treatment completion to distant metastasis was 11.3 months. There were 71, 47, 48, and 34 cases of bone, lung, liver, and other metastases, respectively. Other metastatic sites included the axillary lymph nodes, mediastinal lymph nodes, brain, spleen, adrenal glands, chest wall, and pleura. The median survival post-metastasis was 14 months, and the 1-, 2-, and 3-year survival rates were 60.4%, 40.2%, and 27.6%, respectively (Figure 1).
Prognostic factors
Through univariate analysis, we found that overall survival was related to N staging at diagnosis, whether induction chemotherapy was utilized, time interval from initial radiotherapy completion to distant metastasis (metastatic interval), with/without multi-organ metastasis and liver metastasis, and whether chemotherapy or palliative radiotherapy were utilized after the discovery of metastasis.
Based on Cox regression analysis, the metastatic interval, with/without liver and multi-organ involvement, and whether chemotherapy was utilized were independent prognostic factors for survival. In the multivariate analysis, patients with short metastatic interval, multiple organ metastases, and without local palliative radiotherapy or chemotherapy had a significantly worse prognosis (Figure 2A,B,C,D).

Long-term survival analysis
In total, 21 (13.8%) patients [15 (71.4%) men] survived >3 years after the diagnosis of metastasis, with a median survival of 59.8 months (39.9–103.9 months). The average metastatic interval was 31.4 months and the median interval was 25 months (6–107 months). All patients received treatment for their metastases. Stereotactic body radiotherapy (SBRT) at 48 Gy/6 F was provided for three patients with lung metastasis and two patients with liver metastasis. One patient with liver metastases was treated with radiofrequency ablation. Patients with bone metastases received palliative external beam radiotherapy (radiotherapy dose, 36–60 Gy). All of these long-term survival patients were treated with systemic chemotherapy. The chemotherapy regimens included docetaxel + cisplatin (n=13), gemcitabine + cisplatin (n=5), and cisplatin + fluorouracil+ nimotuzumab (n=3). The median number of chemotherapy cycles for the entire group was 4 (range, 2–6).
Discussion
In recent years, NPC treatment has achieved significant progress in local control rate due to the evolvement of 2-D and 3-D radiotherapy with better dose distribution, as well as the utilization of MRI-based delineation that defines tumor and organs more precisely. The 5-year local area progression-free survival rate for NPC after IMRT treatment was reported at >90% in most cancer centers worldwide (15,16). IMRT significantly improved the local area control rate; however, this did not translate into improved patient survival due to the occurrence of distant metastases, which pose a challenge for patients with NPC (2-5). In this study, we investigated the characteristics and prognostic factors of distant metastatic NPC after IMRT and provided our experience for appropriate salvage therapies to improve the overall survival rate of NPC.
Various survival rates for patients with distant metastatic NPC have been reported in previous studies. The median survival time and 2-year survival rate ranges from 9–15.6 months and 15.0–34.4%, respectively. The survival rate may be related to whether the patients received treatment, as well as the treatment methods and intensity in the various studies (17-20). Based on our findings, the median survival was 14 months, and the post-metastasis1-, 2-, and 3-year survival rates were 60.4%, 40.2%, and 27.6%, respectively. The most common metastatic sites were the bones, lungs, and the liver. The 2-year survival rate observed in this study was higher than that previously reported. There are two possible reasons for the discrepancies between our findings and those previously reported. Firstly, although this was a retrospective study, the treatment for all patients was selected by a multidisciplinary team (MDT), based on the patients’ disease status and physical condition. Thus, there was no bias between different physicians regarding treatment options. Hence, our results are a good representation of the general situation of patients with metastatic NPC after IMRT. Secondly, most of the patients received local radiotherapy after disease remission or control by systemic chemotherapy. Radiotherapy eliminated local residual lesions that were not sensitive or resistant to chemotherapy. Furthermore, the combination of radiotherapy and chemotherapy reduced the overall tumor load and delayed the progression of the disease.
The N stage at initial treatment independently affects the survival of patients with metastatic NPC (15). However, although induction chemotherapy and the N stage at initial diagnosis had effects on survival after metastasis (determined via univariate analysis), these factors were not found to be significant independent prognostic factors by multivariate analysis. This could be explained by the fact that 71.1% of patients had N2 or N3 stages at initial diagnosis. Relatively high-risk N2 (>3 cm lymph node or with fusion necrosis) and N3 stage patients were provided 3–6 full cycles of treatment, in conjunction with an adequate amount of induction chemotherapy, which may have balanced the N-stage effects and improved the prognosis of high-risk N stage patients. Therefore, the N-stage did not have a critical impact on patient survival in the current study.
The findings of the multivariate analysis in this study were similar to those reported in previous studies (17-19). Prognosis can vary according to the different metastatic sites. The median survival time of intrahepatic metastases was only 10.5 months, while that of extrahepatic metastases was 16 months. The median survival times of bone and lung metastases were 18 and 16 months, respectively, while that of multi-organ metastases was only 10 months. The survival rate of multiple organ metastases was significantly lower than that of any single organ metastasis. Previous studies have reported that patients with only lung or bone metastases have a better prognosis and long-term survival (17,18), however no clear comparisons have been made in respective studies.
Local palliative therapy is a very important method for the treatment of metastatic NPC. For patients with bone metastases, radiotherapy after chemotherapy, combined with bisphosphonate treatment to inhibit osteoclast activity, can effectively relieve pain and prevent fractures. In patients with only lung metastases, chemotherapy combined with targeted therapy is an effective treatment option. After 4–6 cycles of chemotherapy, local radiotherapy is performed for intrapulmonary metastatic lesions. For a single lung metastatic lesion or multiple lesions in a single lobe, surgery or SBRT could also be considered if the patient is in good health. Liver metastasis generally involves multiple lesions and therefore, performing surgery is difficult. The liver poorly tolerates radiation; therefore, chemotherapy has always been used as the preferred treatment option. Patients with oligometastatic lesions can also consider SBRT or radiofrequency ablation.
In the current study, 21 patients survived for more than 3 years after the diagnosis of metastasis, with a median survival of 59.8 months (39.9–103.9 months). All metastatic lesions were treated with radical radiotherapy. SBRT for metastatic lesions was performed in three patients with lung metastases and two patients with liver metastases. Furthermore, one patient with liver metastasis was treated with radiofrequency ablation. All patients had long-term survival of >3 years. It is suggested that there is a great possibility of cure for nasopharyngeal carcinoma with oligometastases after radical chemoradiotherapy combined with local radical therapy.
Systemic chemotherapy is the preferred treatment choice for metastatic NPC (6,7). In this study, the median survival time of patients who received systemic chemotherapy was 18 months, which significantly differed from that of patients who did not receive chemotherapy (9 months). However, the efficacy of chemotherapy may depend on various patient factors. Previous research has shown that factors such as the general condition of patients, hemoglobin levels, and body weight can affect the treatment efficacy of metastatic NPC. The efficacy of different chemotherapy regimens can also vary. According to the results of multiple phase II clinical studies, the combination of two or three platinum-containing first-line drugs can achieve an efficiency of 50–80%, and a median progression-free and overall survival of 5–11 and 10–14 months, respectively (21-27). The metastatic NPC chemotherapy regimens used in this study were docetaxel + cisplatin, gemcitabine + cisplatin, or cisplatin + fluorouracil + nimotuzumab. There was no difference in the efficacy between these three regimens. However, prospective randomized controlled trials of these chemotherapy regimens are limited, and therefore, prospective clinical trials are needed to compare the efficacy between chemotherapy regimens. We did not identify a prognostic effect of the targeted drugs by univariate analysis; this may be due to the small number of patients (n=14) who underwent chemotherapy in addition to targeted therapy. Hence, this finding may not reflect the true efficacy of targeted drugs used for metastatic NPC.
There were several limitations in the present study that should be noted. Firstly, the copy number of serum Epstein-Barr virus deoxyribonucleic acid (DNA) is an important prognostic factor in patients with NPC (28). However, only a small number of patients in this study had the pre-treatment serum Epstein-Barr virus DNA copy number and therefore, it was not included in the prognostic analysis of the patients.
In conclusion, the treatment of metastatic NPC remains challenging. Although treatment has improved, the overall prognosis of patients remains poor. Presently, systemic chemotherapy with a platinum-based regimen remains an important first-line treatment. The combined application of localized radiotherapy and systemic chemotherapy requires further investigation. We found that there was a potential for cure or long-term remission after 4–6 cycles of chemotherapy and active local treatment of metastases in patients with only bone or lung metastases who were in good general health. For patients with multiple liver metastases or multiple organ metastases who had poor general health, the median survival time was relatively short, and thus, the best supportive care should be provided. The findings from this study can assist clinicians in more accurately determining the prognosis of patients with metastatic NPC. Additionally, they might provide guidance on the selection of individualized treatment plans, which can prevent excessive or ineffective treatment for patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-21-1279
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-21-1279
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-21-1279). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The present study was approved by the Ethics Committee of the First Affiliated Hospital of Air Force Medical University (ethical approval number: KY20172007-1). All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev 2006;15:1765-77. [Crossref] [PubMed]
- Sanguineti G, Geara FB, Garden AS, et al. Carcinoma of the nasopharynx treated by radiotherapy alone: determinants of local and regional control. Int J Radiat Oncol Biol Phys 1997;37:985-96. [Crossref] [PubMed]
- Vikram B, Mishra UB, Strong EW, et al. Patterns of failure in carcinoma of the nasopharynx: I. Failure at the primary site. Int J Radiat Oncol Biol Phys 1985;11:1455-9. [Crossref] [PubMed]
- Lee N, Xia P, Quivey JM, et al. Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: an update of the UCSF experience. Int J Radiat Oncol Biol Phys 2002;53:12-22. [Crossref] [PubMed]
- Kwong DL, Sham JS, Leung LH, et al. Preliminary results of radiation dose escalation for locally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2006;64:374-81. [Crossref] [PubMed]
- Ji JH, Yun T, Kim SB, et al. A prospective multicentre phase II study of cisplatin and weekly docetaxel as first-line treatment for recurrent or metastatic nasopharyngeal cancer (KCSG HN07-01). Eur J Cancer 2012;48:3198-204. [Crossref] [PubMed]
- Chen C, Wang FH, An X, et al. Triplet combination with paclitaxel, cisplatin and 5-FU is effective in metastatic and/or recurrent nasopharyngeal carcinoma. Cancer Chemother Pharmacol 2013;71:371-8. [Crossref] [PubMed]
- Lin S, Tham IW, Pan J, et al. Combined high-dose radiation therapy and systemic chemotherapy improves survivalin patients with newly diagnosed metastatic nasopharyngeal cancer. Am J Clin Oncol 2012;35:474-9. [Crossref] [PubMed]
- Zeng L, Tian YM, Huang Y, et al. Retrospective analysis of 234 nasopharyngeal carcinoma patients with distant metastasis at initial diagnosis: therapeutic approaches and prognostic factors. PloS One 2014;9:e108070 [Crossref] [PubMed]
- Chen MY, Jiang R, Guo L, et al. Locoregional radiotherapy in patients with distant metastases of nasopharyngeal carcinoma at diagnosis. Chin J Cancer 2013;32:604-13. [Crossref] [PubMed]
- Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006;354:567-78. [Crossref] [PubMed]
- Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 2008;359:1116-27. [Crossref] [PubMed]
- Cohen EE, Kane MA, List MA, et al. Phase II trial of gefitinib 250 mg daily in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res 2005;11:8418-24. [Crossref] [PubMed]
- Soulieres D, Senzer NN, Vokes EE, et al. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol 2004;22:77-85. [Crossref] [PubMed]
- Sultanem K, Shu HK, Xia P, et al. Three-dimensional intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: the University of California-San Francisco experience. Int J Radiat Oncol Biol Phys 2000;48:711-22. [Crossref] [PubMed]
- Parliament MB, Scrimger RA, Anderson SG, et al. Preservation of oral health-related quality of life and salivary flow rates after inverse-planned intensity- modulated radiotherapy (IMRT) for head-and-neck cancer. Int J Radiat Oncol Biol Phys 2004;58:663-73. [Crossref] [PubMed]
- Khanfir A, Frikha M, Ghorbel A, et al. Prognostic factors in metastatic nasopharyngeal carcinoma. Cancer Radiother 2007;11:461-4. [Crossref] [PubMed]
- Teo PM, Kwan WH, Lee WY, et al. Prognosticators determining survival subsequent to distant metastasis from nasopharyngeal carcinoma. Cancer 1996;77:2423-31. [Crossref] [PubMed]
- Fandi A, Bachouchi M, Azli N, et al. Long-term disease-free survivors in metastatic undifferentiated carcinoma of nasopharyngeal type. J Clin Oncol 2000;18:1324-30. [Crossref] [PubMed]
- King AD, Ma BB, Yau YY, et al. The impact of 18F-FDG PET/CT on assessment of nasopharyngeal carcinoma at diagnosis. Br J Radiol 2008;81:291-8. [Crossref] [PubMed]
- Chua DT, Yiu HH, Seetalarom K, et al. Phase II trial of capecitabine plus cisplatin as first-line therapy in patients with metastatic nasopharyngeal cancer. Head Neck 2012;34:1225-30. [Crossref] [PubMed]
- Yi J, Huang X, Gao L, et al. Intensity-modulated radiotherapy with simultaneous integrated boost for locoregionally advanced nasopharyngeal carcinoma. Radiat Oncol 2014;9:56. [Crossref] [PubMed]
- Ma SX, Zhou T, Huang Y, et al. The efficacy of first-line chemotherapy in recurrent or metastatic nasopharyngeal carcinoma: a systematic review and meta-analysis. Ann Transl Med 2018;6:201. [Crossref] [PubMed]
- Hsieh JC, Hsu CL, Ng SH, et al. Gemcitabine plus cisplatin for patients with recurrent or metastatic nasopharyngeal carcinoma in Taiwan: a multicenter prospective Phase II trial. Jpn J Clin Oncol 2015;45:819-27. [Crossref] [PubMed]
- Zhang L, Huang Y, Hong S, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet 2016;388:1883-92. [Crossref] [PubMed]
- Long GX, Lin JW, Liu DB, et al. Single-arm, multi-centre phase II study of lobaplatin combined with docetaxel for recurrent and metastatic nasopharyngeal carcinoma patients. Oral Oncol 2014;50:717-20. [Crossref] [PubMed]
- Huang Y, Liang W, Yang Y, et al. Phase I/II dose-finding study of nanoparticle albumin-bound paclitaxel (nab(R)-Paclitaxel) plus Cisplatin as Treatment for Metastatic Nasopharyngeal Carcinoma. BMC Cancer 2016;16:464. [Crossref] [PubMed]
- Gamba P, Rota L, Abeni C, et al. Integrated Diagnostic Model That Incorporates Epstein-Barr Virus DNA, Imaging, and Nasal Endoscopy to Stratify Primary Tumor and Lymph Nodes in a Patient with N1 Nasopharyngeal Carcinoma: Multidisciplinary Management. Case Rep Oncol 2018;11:289-97. [Crossref] [PubMed]



