
Systematic review and meta-analysis of the efficacy of trypsin inhibitors in patients with severe pancreatitis
Introduction
Acute pancreatitis (AP) is a common pancreatic disease, and is the result of a combination of several factors (1). During an AP attack, a platelet-activating factor is released, which causes damage and necrosis of the intestinal mucosa. AP is characterized by rapid onset and leads to organ dysfunction, with a case fatality rate as high as 35% (2). Severe pancreatitis (SP) accounts for approximately 25% of AP patients, and manifests as pancreatic necrosis, pancreatic abscesses, and cysts on the basis of AP, and is often accompanied by infection and shock (3).
In SP patients, trypsin is activated inside the pancreas, damaging pancreatic cells. Trypsin further activates lipase, phospholipase, and elastase, resulting in pancreatic tissue digesting itself (4). When pancreatic cells are injured, a variety of inflammatory mediators will be produced to activate the tumor necrosis factor-α (TNF-α) signaling pathway, involving the kidney, lung, and other organs, thereby triggering systemic inflammation (5). At this stage, non-surgical treatment is adopted to prevent the activation of protease, so as to improve micro-circulation, keep the internal circulation stable, maintain the hemodynamics of the entire body, and enhance the body’s stress ability (6).
Trypsin inhibitor generally refers to aprotinin, which can inhibit trypsin and chymotrypsin, as well as prevent the activation of other active proteases in the pancreas and the self-activation of trypsinogen (7). Clinically, it is used to prevent and treat AP, and also for anti-shock therapy. As for other types of trypsin inhibitors, they can inhibit the activity of corresponding proteases and are also used in the treatment of pancreatitis (8). Trypsin inhibitors can not only oxidize free radicals, but also reduce the activity of inflammation, effectively alleviating the symptoms of SP and reducing inflammation (9). Common trypsin inhibitors include ulinastatin, somatostatin, and octreotide (10).
Zhai et al. [2019] (11) reported that the cause of SP infection is attributable to the damage of the intestinal mucosal epithelial tissue, which increases the permeability of the intestine, so that bacteria are transferred from the intestine to the blood. Despite an increasing number of randomized controlled trials (RCTs) involving SP patients, these studies have distinct efficacy outcomes, and the number of samples in each study is limited. Consequently, it is currently impossible to confirm the efficacy of trypsin inhibitors in treatment of SP. In this study, meta-analysis was employed for scientific evaluation of the included RCTs, in order to assess the clinical efficacy of trypsin inhibitors in the treatment of SP patients.
We present the following article in accordance with the PRISMA reporting checklist (available at https://dx.doi.org/10.21037/apm-21-1206).
Methods
Literature retrieval
The PubMed, Medline, Cochrane Library, Chinese Biomedical Literature, CNKI, WANFANG, VIP, and Google Scholar databases were used to retrieve relevant literature from the date of establishment of the database to December 20th, 2020. The Boolean logic retrieval method was used to retrieve relevant references. Chinese and English databases were respectively searched a combination of the following search terms: “trypsin inhibitor”, “acute pancreatitis”, and “severe pancreatitis”. The quality of the literature was evaluated according to the RevMan 5.3 software provided by the Cochrane system.
Each database adopted a joint search strategy of free words and subject words. After confirmation, the references were tracked using the search engine, and the latest research progress was obtained after contacting experts and researchers in the field.
Literature inclusion and exclusion criteria
Inclusion criteria: (I) related studies on SP treatment by trypsin inhibitors; (II) RCTs; (III) studies that included a pathological control analysis showing that the index was reliable in the 95% confidence interval (CI); (IV) studies published in Chinese or English; (V) the diagnosis of SP met the standards of the World Health Organization.
Exclusion criteria: (I) irrelevant literature; (II) repeatedly literature published; (III) literature reviews, abstracts, case reports, or animal experiments; (IV) studies where the complete data could not be obtained; (V) studies published in languages other than Chinese or English.
Two senior experts independently screened the abstracts and full texts of the relevant studies, and three preliminary experiments were performed prior to screening. Inconsistencies between experts were resolved through discussion and consensus, or a third expert could be invited to arbitrate.
Literature screening
Literature screening was divided into three steps. Studies that were not related to this research were initially excluded by reading the titles and abstracts. Studies that did not meet the requirements were subsequently excluded by reading the full texts. The quality evaluation was then performed. Two senior experts independently screened the abstracts and full texts of the relevant literature, and three preliminary experiments were performed prior to screening. Inconsistencies between experts were resolved through discussion and consensus, or a third expert could be invited to arbitrate.
Data extraction
Two experts used a unified Microsoft Excel table to independently extract relevant data. Three preliminary experiments were performed prior to data extraction. Inconsistencies between experts were resolved through discussion and consensus, or a third expert could be invited to arbitrate. Extracted data included: (I) the title of the research; (II) the first author and the year of publication; (III) the name of the publication; (IV) the publication time of the research; (V) the basic information of the research subject (average age, gender, treatment plan, and drug dosage); (VI) the grouping and statistical methods of the experimental and control groups; (VII) the source, sample size, and outcome indexes of the cases.
Quality evaluation
Two researchers simultaneously conducted a risk of bias assessment. Inconsistencies between experts were resolved through discussion and consensus, or a third expert could be invited to arbitrate. The quality evaluation of this study referred to the JADAD score in the Cochrane Systematic Review Manual, and the evaluation content included the following: whether a RCT was adopted; whether the random method was described properly; whether the blinding method was used correctly; whether the baseline data of the literature was similar and compliant; and whether the reason for the patient’s withdrawal was accurately described. A score of 0–2 was considered low-quality research, a score of 3–5 was considered medium-quality research, and a score of 6–9 was considered high-quality research.
Risk of bias assessment
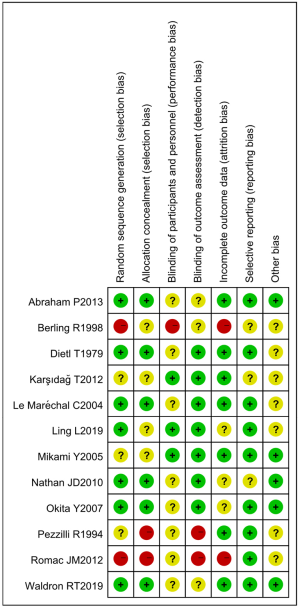
Two researchers simultaneously conducted a risk of bias assessment. Inconsistencies between experts were resolved through discussion and consensus, or a third expert could be invited to arbitrate. In this study, the Cochrane Collaboration tool was for “bias risk assessment” of the included RCTs. Judgments of “low risk of bias”, “unclear”, and “high risk of bias” were made according to the following five aspects: random allocation method, blind method, allocation concealment, complete data, and research results.
Statistical methods
Stata SE 12.0 software was used for statistical analysis. The risk ratio (RR) and 95% CI were used to evaluate the rates of pancreatic infection, extra-pancreatic infection, and mortality in the treatment of SP patients by trypsin inhibitor, and the mean difference (MD) 95% CI was used to evaluate the length of hospital stay and TNF-α level. RevMan 5.3 software was used to assess the risk of bias of the included studies, and the effect was expressed using a 95% CI. When P>0.1 and I2<50%, the fixed effects model was used for meta-analysis, whereas when P<0.1 and I2>50%, the random effects model was used.
Results
The basic information of included references
Initially, a total of 1,692 references were identified. Then, 47 were eliminated after reading the title, and 1,261 were further eliminated after reading the abstract. Of the 384 remained, 337 were eliminated for duplicate subjects after reading the full text, and 35 were eliminated for not being the RCTs on anesthesia surgery for liver cancer. Finally, 12 references were identified for meta-analysis (Figure 1).
Figure 2 shows the quality classification results. It was evident that there were six studies with a score of 6–9 and above, four studies with a score of 3–5, and two studies with a score of 0–2.
Twelve references met the inclusion criteria, including eight retrospective analyses and four of RCTs, involving a total of 823 cases. The experimental group had received trypsin inhibitor treatment, and no treatment was provided to the control group. The 12 references all belonged to small sample studies (sample sizes ranging from 47 to 92), and they were analyzed for number of cases, intervention measures, and observation indexes. The general information of the included references is shown in Table 1.
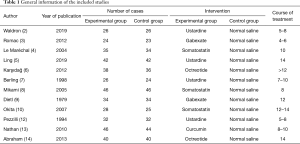
Full table
Risk of bias evaluation
Figures 3,4 show the multiple risk of bias evaluation results of the included studies using the RevMan 5.3 software. Of the 12 RCTs included in this study, two (12,13) had the correct random allocation method, and only one (14) had detailed allocation concealment. One (15) reference was evaluated using the blinding method, which was not adopted in the other references. However, the measurement indexes in this study were laboratory indexes determined by computer. Hence, it can be considered that all of the included studies used the blinding method correctly.
Pancreatic infection
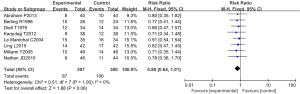
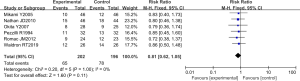
Eight RCTs analyzed the incidence of pancreatic infection. There were 607 cases in total, with 307 cases in the experimental group and 300 cases in the control group. The heterogeneity test results were Chi2 =0.51, degrees of freedom (df) =7, I2=0%<50%, and P=1.00>0.01, and the fixed effects model was used for analysis. The horizontal line and the invalid vertical line crossed in the 95% CI of all studies. The meta-analysis results showed that the incidence of pancreatic infection in the experimental group was lower than that in the control group, with a RR: 0.80 and a 95% CI: (0.64, 1.01). However, the difference was not statistically significant (Z=1.88, P=0.06) (Figure 5).
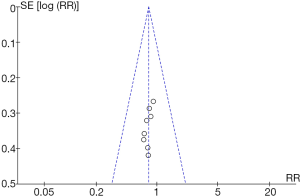
RevMan 5.3 was used to obtain a funnel chart of pancreatic infection (Figure 6). It was noted that the circles in some studies were basically symmetrical to along the midline, suggesting that the research accuracy was high and that there was no publication bias.
The incidence of extra-pancreatic infection
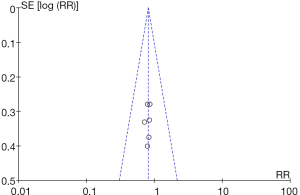
Six RCTs analyzed the incidence of extra-pancreatic infection. There were 396 cases in total, with 202 cases in the experimental group and 196 cases in the control group. The heterogeneity test results were Chi2 =0.20, df =5, I2=0%<50%, and P=1.00>0.01, and the fixed effects model was used for analysis. The horizontal line and the invalid vertical line crossed in the 95% CI of all studies. The meta-analysis results showed that the incidence of extra-pancreatic infection in the experimental group was lower than that in the control group, with a RR: 0.81 and 95% CI: (0.62, 1.05), but the difference was not statistically significant (Z=1.60, P=0.11) (Figure 7).
RevMan 5.3 was used to obtain a funnel chart of extra-pancreatic infection (Figure 8). It was noted that the circles in some studies were basically symmetrical to along the midline, suggesting that the research accuracy was high and that there was no publication bias.
Length of hospital stay
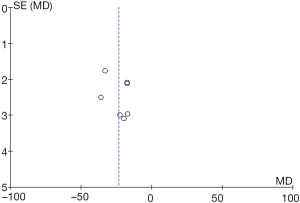
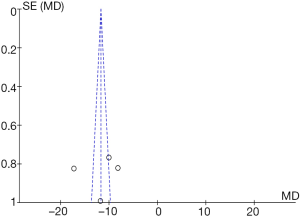
Seven RCTs analyzed the length of hospital stay. There were 530 cases in total, with 269 in the experimental group and 261 in the control group. The heterogeneity test results were Tau2 =65.69, Chi2 =75.05, df =6, I2=92%>50%, and P<0.0001, and the random effects model was used for analysis. The horizontal line was on the left of the invalid vertical line in the 95% CI of all studies. The meta-analysis results showed that the length of hospital stay of the experimental group was shorter than that of the control group, with MD: –23.31 and the 95% CI: (–29.60, –17.02), and the difference was statistically significant (Z=7.26, P<0.0001) (Figure 9).

RevMan 5.3 was used to obtain a funnel chart of the length of hospital stay (Figure 10). It was noted that the circles in some studies were basically symmetrical to along the midline, suggesting that the research accuracy was high and that there was no publication bias.
TNF-α level
Four RCTs analyzed the level of inflammatory factor TNF-α. There were 335 cases in total, with 169 in the experimental group and 166 in the control group. The heterogeneity test results were Chi2 =67.28, df =3, I2=96%>50%, and P<0.0001, and the random effects model was used for analysis. The horizontal line was on the left of the invalid vertical line in the 95% CI of all studies. The meta-analysis results showed that the level of TNF-α in the experimental group was lower than that in the control group, with MD: –11.69, 95% CI: (–12.51, –10.87), and the difference was statistically significant (Z=27.88, P<0.0001) (Figure 11).
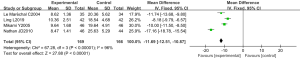
RevMan 5.3 was used to obtain a funnel chart of TNF-α level (Figure 12). It was noted that the circles in some studies were basically symmetrical to along the midline, suggesting that the research accuracy was high and that there was no publication bias.
Mortality rate
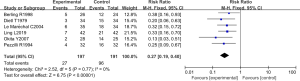
Six RCTs analyzed the mortality rate. There were 388 cases in total, with 197 in the experimental group and 191 in the control group. The heterogeneity test results were Chi2 =2.52, df =5, I2=0%<50%, and P=0.77>0.01, and the fixed effects model was used for analysis. The horizontal line was on the left of the invalid vertical line in the 95% CI of all studies. The meta-analysis results showed that the mortality rate of the experimental group was lower than that of the control group, with a RR: 0.27 and a 95% CI: (0.19, 0.40), and the difference was statistically significant (Z=6.75, P<0.0001) (Figure 13).
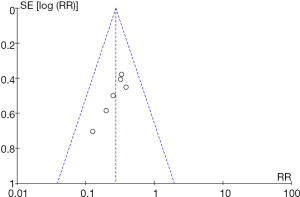
RevMan 5.3 was used to obtain a funnel chart of mortality rate (Figure 14). It was noted that the circles in some studies were basically symmetrical to along the midline, suggesting that the research accuracy was high and that there was no publication bias.
Discussion
Trypsin inhibitors can inhibit the secretion and activation of trypsin in SP patients (16). Normally, the digestive enzymes in pancreatic cells are in the form of zymogen. Under normal internal conditions, trypsin can be present in pancreatic cells as inactive trypsinogen (17). During an SP attack, trypsin will be activated, damaging pancreatic cells and small blood vessel walls, resulting in the release of inflammatory factors. As a result, vascular permeability will increase, and pancreatic tissue will experience edema, hemorrhage, and even necrosis (18). In this study, meta-analysis was used to evaluate the efficacy of trypsin inhibitors in the treatment of SP patients. The quality classification results showed that there were six included studies with a score of 6–9 and above, four with a score of 2–5, and two with a score below 2. Among the 12 included RCTs, two had the correct random allocation method, with only one having detailed allocation concealment.
The trypsin inhibitor group and the blank control group were compared in terms of pancreatic infection, extra-pancreatic infection, length of hospital stay, TNF-α level, and mortality rate. Eight RCTs analyzed the incidence of pancreatic infection. There were 607 cases in total, with 307 cases in the experimental group and 300 cases in the control group. The heterogeneity test results were Chi2 =0.51, df =7, I2=0%<50%, and P=1.00>0.01. The incidence of pancreatic infection in the experimental group was lower than that in the control group, with a RR: 0.80, 95% CI: (0.64, 1.01), Z=1.88, and P=0.06.
Six RCTs analyzed the incidence of extra-pancreatic infection. There were 396 cases in total, with 202 cases in the experimental group and 196 cases in the control group. The heterogeneity test results were Chi2 =0.20, df =5, I2=0%<50%, and P=1.00>0.01. The incidence of extra-pancreatic infection in the experimental group was lower than that in the control group, with a RR: 0.81, 95% CI: (0.62, 1.05), Z=1.60, and P=0.11.
Seven RCTs analyzed the length of hospital stay. There were 530 cases in total, with 269 in the experimental group and 261 in the control group. The heterogeneity test results were Tau2 =65.69, Chi2 =75.05, df =6, I2=92%>50%, and P<0.0001. The length of hospital stay of the experimental group was shorter than that of the control group, with a MD: –23.31 and 95% CI: (–29.60, –17.02), and the difference was statistically significant (Z=7.26, P<0.0001).
Four RCTs analyzed the level of inflammatory factor TNF-α. There were 335 cases in total, with 169 in the experimental group and 166 in the control group. The heterogeneity test results were Chi2= 67.28, df =3, I2=96%>50%, and P<0.0001. The level of TNF-α in the experimental group was lower than that in the control group, with a MD: –11.69 and 95% CI: (–12.51, –10.87), and the difference was statistically significant (Z=27.88, P<0.0001).
Six RCTs analyzed the mortality rate. There were 388 cases in total, with 197 in the experimental group and 191 in the control group. The heterogeneity test results were Chi2 =2.52, df =5, I2=0%<50%, and P=0.77>0.01. The mortality rate of the experimental group was lower than that of the control group, with a RR: 0.27 and 95% CI: (0.19, 0.40), and the difference was statistically significant (Z=6.75, P<0.0001).
The above results were consistent with the research results of Lagoo et al. [2018] (19). RevMan 5.3 was used to obtain the funnel charts of each observation index. It was evident that the circles in some studies were basically symmetrical along the midline, which indicated that the research accuracy was high and that there was no publication bias.
Conclusions
In this study, the Boolean logic search method was used to identify 12 studies, with the trypsin-treated SP group as the experimental group and the blank control as the control group. Meta-analysis was conducted to explore the efficacy of trypsin in SP patients, and confirmed that trypsin inhibitors can inhibit the release of inflammatory factors and reduce the mortality rate of patients. However, some limitations of this should be noted. The included studies had low quality and high heterogeneity, and the sample size was small. Hence, an expanded sample size is necessary in follow-up clinical RCTs to further verify our findings. In conclusion, the results of this study provide a reliable theoretical basis for the clinical treatment of SP.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://dx.doi.org/10.21037/apm-21-1206
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apm-21-1206). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rainio M, Lindström O, Penttilä A, et al. Serum serine peptidase inhibitor Kazal-type 1, trypsinogens 1 to 3, and complex of trypsin 2 and α1-antitrypsin in the diagnosis of severe acute pancreatitis. Pancreas 2019;48:374-80. [Crossref] [PubMed]
- Waldron RT, Chen Y, Pham H, et al. The Orai Ca2+ channel inhibitor CM4620 targets both parenchymal and immune cells to reduce inflammation in experimental acute pancreatitis. J Physiol 2019;597:3085-105. [Crossref] [PubMed]
- Romac JM, Shahid RA, Choi SS, et al. Pancreatic secretory trypsin inhibitor I reduces the severity of chronic pancreatitis in mice overexpressing interleukin-1β in the pancreas. Am J Physiol Gastrointest Liver Physiol 2012;302:G535-41. [Crossref] [PubMed]
- Le Maréchal C, Chen JM, Le Gall C, et al. Two novel severe mutations in the pancreatic secretory trypsin inhibitor gene (SPINK1) cause familial and/or hereditary pancreatitis. Hum Mutat 2004;23:205. [Crossref] [PubMed]
- Ling L, Li Y, Li H, et al. MMP-2 and MMP-9 gene polymorphisms act as biological indicators for ulinastatin efficacy in patients with severe acute pancreatitis. Medicine (Baltimore) 2019;98:e15831 [Crossref] [PubMed]
- Karşıdağ T, Tüzün S, Kemik AS, et al. Alpha-1 protease inhibitor and antichymotrypsin levels in acute pancreatitis. Ulus Travma Acil Cerrahi Derg 2012;18:195-9. [Crossref] [PubMed]
- Berling R, Borgström A, Ohlsson K. Peritoneal lavage with aprotinin in patients with severe acute pancreatitis. Effects on plasma and peritoneal levels of trypsin and leukocyte proteases and their major inhibitors. Int J Pancreatol 1998;24:9-17. [PubMed]
- Mikami Y, Takeda K, Matsuda K, et al. Rat experimental model of continuous regional arterial infusion of protease inhibitor and its effects on severe acute pancreatitis. Pancreas 2005;30:248-53. [Crossref] [PubMed]
- Dietl T, Dobrinski W, Hochstrasser K. Human inter-alpha-trypsin inhibitor. Limited proteolysis by trypsin, plasmin, kallikrein and granulocytic elastase and inhibitory properties of the cleavage products. Hoppe Seylers Z Physiol Chem 1979;360:1313-8. [Crossref] [PubMed]
- Okita Y, Okahisa T, Sogabe M, et al. Low-volume continuous hemodiafiltration with nafamostat mesilate increases trypsin clearance without decreasing plasma trypsin concentration in severe acute pancreatitis. ASAIO J 2007;53:207-12. [Crossref] [PubMed]
- Zhai Y, Gan L, Huang S, et al. Therapeutic effect of ultrasound interventional perirenal catheter-assisted early peripancreatic lavage of protease inhibitor on severe acute pancreatitis in miniature pigs. Pancreatology 2019;19:158-62. [Crossref] [PubMed]
- Pezzilli R, Billi P, Platè L, et al. Human pancreatic secretory trypsin inhibitor in the assessment of the severity of acute pancreatitis. A comparison with C-reactive protein. J Clin Gastroenterol 1994;19:112-7. [Crossref] [PubMed]
- Nathan JD, Romac J, Peng RY, et al. Protection against chronic pancreatitis and pancreatic fibrosis in mice overexpressing pancreatic secretory trypsin inhibitor. Pancreas 2010;39:e24-30. [Crossref] [PubMed]
- Abraham P, Rodriques J, Moulick N, et al. Efficacy and safety of intravenous ulinastatin versus placebo along with standard supportive care in subjects with mild or severe acute pancreatitis. J Assoc Physicians India 2013;61:535-8. [PubMed]
- Dugernier T, Laterre PF, Reynaert M, et al. Compartmentalization of the protease-antiprotease balance in early severe acute pancreatitis. Pancreas 2005;31:168-73. [Crossref] [PubMed]
- Emori Y, Mizushima T, Matsumura N, et al. Camostat, an oral trypsin inhibitor, reduces pancreatic fibrosis induced by repeated administration of a superoxide dismutase inhibitor in rats. J Gastroenterol Hepatol 2005;20:895-9. [Crossref] [PubMed]
- Otsuki M, Tani S, Okabayashi Y, et al. Beneficial effects of the synthetic trypsin inhibitor camostate in cerulein-induced acute pancreatitis in rats. Dig Dis Sci 1990;35:242-50. [Crossref] [PubMed]
- Mao X, Yang Z. Current usage status of somatostatin and its analogs and trypsin inhibitors: a real-world study of 34,654 Chinese adult patients with acute pancreatitis. Ann Palliat Med 2021;10:1325-35. [Crossref] [PubMed]
- Lagoo JY, D'Souza MC, Kartha A, et al. Role of Ulinastatin, a trypsin inhibitor, in severe acute pancreatitis in critical care setting: a retrospective analysis. J Crit Care 2018;45:27-32. [Crossref] [PubMed]
(English Language Editor: A. Kassem)





