
Therapeutic effects of Yiqi Huoxue prescription on diabetic nephropathy: a meta-analysis and systematic review
Introduction
With improved living standards, the prevalence of diabetes has shown an increasing trend every year (1,2). Diabetic nephropathy is one of the most common chronic microvascular complications of diabetes, and is also an important cause of death in diabetic patients (3,4). Statistics from the International Diabetes Federation show that deaths due to diabetic nephropathy account for approximately 9% of the total deaths. Hence, there is a pressing need to develop effective treatments for diabetic nephropathy patients (5). At present, the main measures used by Western medicine to treat diabetic nephropathy are to control blood sugar, blood lipids, improve renal function, adjust diet, and exercise moderately (6,7). However, since the pathogenesis of early diabetic nephropathy remains unclear, there is no direct and effective clinical treatment. Chinese medicine combined with Western medicine has achieved good results in the treatment of diabetic nephropathy. The use of Chinese medicine in the treatment of diabetic nephropathy can not only reduce the adverse reactions of patients, but also effectively control the course of the disease (8,9).
Due to the problems of small sample sizes and different research focuses (10,11), it is impossible to effectively make accurate judgments on the therapeutic effects of Yiqi Huoxue prescription in the treatment of early diabetic nephropathy. Hence, to further identify the therapeutic effects of Yiqi Huoxue prescription on diabetic nephropathy, RCTs on using Yiqi Huoxue prescription to treat diabetic nephropathy were selected, involving 9 indicators relating to kidney function and glucose level, and the meta-analysis was performed, which is expected to provide a theoretical basis for the treatment of diabetic nephropathy using Yiqi Huoxue prescription.
We present the following article in accordance with the PRISMA reporting checklist (available at https://dx.doi.org/10.21037/apm-21-1147).
Methods
Literature retrieval
We performed a literature search of the PubMed, Medline, Embase, Web of Sciences, Chinese Biological Medical database, Wanfang, and China Science and Technology Journal (12) databases. The following search terms were used to retrieve relevant articles: “diabetic nephropathy” and “Chinese medicine treatment” or “diabetic nephropathy” and “Qiqi” or “Huoxue” (13).
Literature inclusion and exclusion criteria
Inclusion criteria: (I) the subjects were diabetes patients who met the World Health Organization (WHO) or the American Diabetes Association (ADA) diagnostic criteria (14) or the clinical guidelines for new Chinese medicine; (II) the subjects who met the Mogensen staging criteria, or whose urine microalbumin excretion rate (UAER) was between 30–300 µg/min within 24 hours, or between 20–200 µg/min twice at least in consecutive 6 months; (III) original research reports involving random grouping (experimental and control groups), regardless of whether it was a single-blind, double-blind, or non-blind study; (IV) Yiqi Huoxue prescription treatment was given on the basis of conventional comprehensive treatment, with a therapeutic effects comparison between Yiqi Huoxue prescription combined with Western medicine and Western medicine alone, or between Yiqi Huoxue prescription combined with angiotensin-converting enzyme inhibitor/angiotensin receptor blocker drugs and angiotensin-converting enzyme inhibitor/angiotensin receptor blocker drugs alone; and (V) Yiqi Huoxue prescription were self-made Chinese medicine prescriptions, and the dosage was not limited.
Exclusion criteria: (I) research using other treatment methods as intervention measures; (II) non-original research reports, including reviews, individual case reports, meta-analyses, and treatment experience summaries; (III) pharmacokinetic research literature; (IV) animal test literature; (V) research that did not include a control group or those with poor balance between the groups; and (VI) literature with incomplete information.
Literature screening and outcome index extraction
Two experts independently read and screened the literature. The titles and abstracts were read first, and the literature that obviously did not meet the inclusion criteria was eliminated. Literature that potentially met the requirements was examined carefully to determine whether they should be included. Disagreements between the experts were resolved by a decision made under the discretion of a third expert. The information recorded including the following: first author, year of onset, sample size, sample age, intervention measures, and outcome indexes. Outcome indexes included UAER, 24-h urine protein quantification (24-h UPQ) (15), serum creatinine (SCr), blood urea nitrogen (BUN), fasting blood glucose (FBG), glycosylated hemoglobin (HbA1c), triglycerides (TG), total cholesterol (TC), and total effective rate of treatment.
Literature quality evaluation
The risk of bias assessment tool recommended by the Cochrane System Review Manual was used to evaluate the quality of the included literature. The criteria were as follows: (I) whether the study was a randomized trial; (II) whether there was allocation concealment; (III) whether a blinding method was used; (IV) whether the result data was complete; (V) whether there was selective reporting of the results; and (VI) whether there were other deviations. The revised Jadad Quality Evaluation Scale was used to evaluate the quality of the included literature. The criteria were as follows: (I) the randomized grouping method was employed; (II) whether there was randomization concealment; (III) whether the double-blind method was used; and (IV) whether the patients included in the study withdrew or were lost to follow-up. The total score of the Jadad quality evaluation scale (16) was obtained by adding the above scores, of which 1–3 referred to low-quality literature, and 4–7 referred to high-quality literature.
Funnel plots were used to analyze publication bias. If the two sides of the funnel plot were symmetrical, this indicated that there was no publication bias; otherwise, there was publication bias. The sensitivity analysis of low-quality literature was carried out according to the exclusion method.
Statistical analysis
The Review Manager 5.3 software provided by Cochrane Collaboration Software was used for meta-analysis. The chi-square test was used to evaluate the heterogeneity of the included studies. Heterogeneity test results of P>0.05 indicated that there was no heterogeneity in multiple independent studies, and the fixed effects model was used for analysis. However, heterogeneity analysis result of P≤0.05 indicated that there was heterogeneity in multiple independent studies, and the random effects model was employed for analysis. As for the expression method of measurement data, if each clinical trial adopted the same measurement method, the mean difference (MD) and 95% confidence interval (CI) were used; otherwise, the standard MD (SMD) and 95% CI were applied. Count data were expressed by the 95% CI and odds ratio (OR), relative risk (RR), or rate difference (RD). P<0.05 was set as the threshold for significance.
Results
Literature retrieval and information extraction
A total of 216 reports were initially retrieved from the databases. After duplicates were deleted, 97 abstracts that were potentially relevant to this study were retained. Following careful reading of the abstracts by the experts, 34 articles were finally kept according to the inclusion and exclusion criteria. Of these, 21 studies were excluded due to factors such as non-random, repeated publication, and inability to obtain available data, and 13 randomized controlled studies that satisfied the requirements were finally included. The literature search/selection process is shown in Figure 1, and the basic information of the included research literature is shown in Table 1.
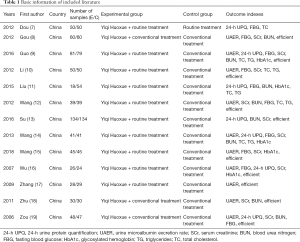
Full table
Quality evaluation of the included literature
The included studies were all randomized controlled trials. However, some of the literature had bias in the random allocation method, whether blind detection was used, whether the data results were complete, etc. As shown in Figures 2 and 3, the risk of bias assessment tool recommended by the Cochrane System Review Manual was utilized.
The Jadad scale was used to evaluate the quality of each included study, as shown in Table 2. The scores of the 13 literatures included in the study were relatively high, and there was no hidden allocation in any of the studies. In three studies, there were patients that dropped out or were lost to follow-up, although reasonable grounds were provided. The overall quality of the literature included in this study was relatively high.
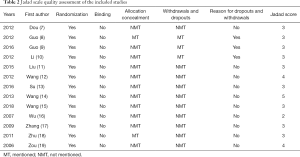
Full table
Analysis of basic data of patients
The age difference between the experimental group and the control group was initially analyzed. After statistical analysis, it was found that there was age heterogeneity between the two groups (I2=77%, P<0.00001). Therefore, the random effects model was used for statistical analysis. As shown in Figure 4, the age meta-analysis result of the two groups was MD (95% CI): −0.30 (−1.18 to 0.58), and the statistical significance test result was Z=0.67. P=0.50. This suggested that there was no notable difference in age between the two groups (P>0.05).
The age bias was compared between the experimental group and the control group. As shown in Figure 5, the age funnel of the two groups was relatively symmetrical and the data was relatively concentrated. Therefore, there was no publication bias for the age of the subjects in the included studies.
Analysis of the patients’ renal function related indexes
The differences in the UAER, 24-h UPQ, SCr, and BUN indexes were analyzed between the experimental and control groups. It was evident from Figure 6A that there was heterogeneity in the UAER levels between the two groups (I2=90%, P<0.00001). After statistical analysis using the random effects model, the meta-analysis result of UAER level was MD (95% CI): −33.94 (−42.60, −25.28), and the result of statistical significance test was Z=7.68, P<0.00001. This showed that the UAER levels of the experimental group were notably lower than the control group (P<0.05).
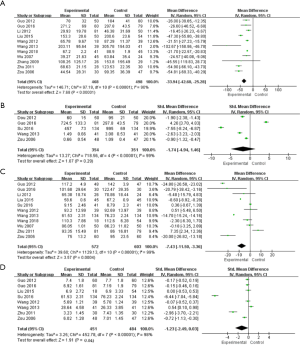
The methods for detecting 24-h UPQ varied among the included studies. Therefore, the SMD was used for analysis. It was evident from Figure 6B that there was heterogeneity in 24-h UPQ levels between the two groups (I2=99%, P<0.00001). After statistical analysis using the random effects model, the meta-analysis result of the patients’ 24-h UPQ levels was SMD (95% CI): −1.74 (−4.94, 1.46), and the statistical significance test result was Z=1.07, P=0.29. This illustrated that the 24-h UPQ levels of the experimental group were not notably different from those of the control group (P>0.05).
Furthermore, it was evident from Figure 6C that there was heterogeneity in the SCr levels of the two groups (I2=99%, P<0.00001). After statistical analysis using the random effects model, the meta-analysis result of SCr levels was MD (95% CI): −7.43 (−11.50, −3.36), and the result of statistical significance test was Z=3.57, P=0.0004. This showed that the SCr level of the experimental group was notably lower than that of the control group (P<0.05).
The methods for detecting BUN varied among the included studies. Hence, the SMD was used for analysis. It was evident from Figure 6D that there was heterogeneity in the BUN levels of the two groups (I2=98%, P<0.00001). After statistical analysis using the random effects model, the meta-analysis result of the BUN level was SMD (95% CI): −1.23 (−2.49, 0.03), and the statistical significance test result was Z=1.91, P=0.04. This indicated that the BUN levels of the experimental group were notably lower than those of the control group (P<0.05).
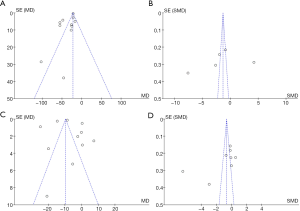
The bias of the renal function indexes (UAER, 24-h UPQ, SCr, and BUN) between the experimental and control groups was analyzed. As shown in Figure 7, the funnel diagrams of UAER, 24-h UPQ, SCr, and BUN of the two groups were symmetrical, and the data was relatively concentrated. Only a few samples were not included. Therefore, the renal function indexes (UAER, 24-h UPQ, SCr, and BUN) did not exhibit large publication bias.
Analysis of patients’ blood glucose- and blood lipid-related indexes
The differences between the blood glucose-related indexes (FBG and HbA1c levels) and the blood lipid-related indexes (TC and TG levels) between the experimental group and the control group were analyzed. It was evident from Figure 8A that there was heterogeneity in the FBG levels of the two groups (I2=83%, P<0.00001). After statistical analysis using the random effects model, the meta-analysis result of FBG level was MD (95% CI): −0.43 (−0.87, 0.01), and the result of statistical significance test was Z=1.90, P=0.03. This showed that the FBG levels in the experimental group were notably lower than those in the control group (P<0.05).
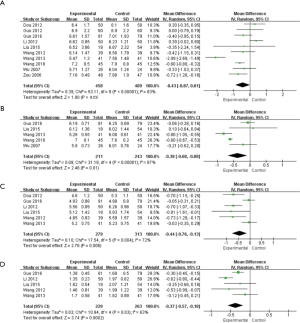
It was also evident from Figure 8B that there was heterogeneity in the HbA1c levels of the two groups (I2=87%, P<0.00001). After statistical analysis using the random effects model, the meta-analysis result of HbA1c level was MD (95% CI): −0.38 (−0.68, −0.08), and the result of statistical significance test was Z=2.46, P=0.01. This showed that the HbA1c levels of the experimental group were notably lower than those of the control group (P<0.05).
As shown in Figure 8C, there was heterogeneity in the TC level of the two groups (I2=72%, P=0.004). After statistical analysis using the random effects model, the meta-analysis result of TC level was MD (95% CI): −0.44 (−0.76, −0.13), and the result of statistical significance test was Z=2.76, P=0.006. This showed that the TC levels of the experimental group were notably lower than those of the control group (P<0.05).
Figure 8D shows that there was heterogeneity in the TG levels of the two groups (I2=63%, P=0.03). After statistical analysis using the random effects model, the meta-analysis result of TG level was MD (95% CI): −0.37 (−0.57, −0.18), and the result of statistical significance test was Z=3.74, P=0.0002. This showed that the TG levels of the experimental group were notably lower than those of the control group (P<0.05).
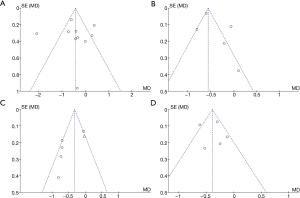
The publication bias of blood glucose-related indexes (FBG and HbA1c levels) and blood lipid-related indexes (TC and TG levels) in the two groups was also analyzed. As shown in Figure 9, the funnel diagram of blood glucose-related indexes (FBG and HbA1c levels) and blood lipid-related indexes (TC and TG) was relatively symmetrical, and only a few samples were not included. Therefore, there was no publication bias in the patients’ blood glucose-related indexes (FBG and HbA1c levels) and blood lipid-related indexes (TC and TG) in the included studies.
Analysis of treatment effectiveness rate
The total effectiveness rate of the experimental and control groups were compared. It was evident from Figure 10 that there was no heterogeneity in the treatment effective rate of the two groups (I2=0%, P=0.91). After statistical analysis using a fixed effects model, the meta-analysis result of the treatment effectiveness rate was OR (95% CI): 3.81 (2.71, 5.35), and the statistical significance test result was Z=7.73, P<0.00001. This showed that the treatment effectiveness rate of the experimental group was notably higher than that of the control group (P<0.05).
The publication bias in the treatment effectiveness rate between the experimental and control groups was analyzed. As shown in Figure 11, the funnel diagram of the treatment effectiveness rate of the two groups was symmetrical, and the data was concentrated in the funnel diagram, indicating that the literature included in this analysis had no bias in the patient treatment effectiveness rate.
Discussion
Traditional Chinese medicine practitioners believe that diabetic nephropathy is similar to “Xiao Ke” (clinical stage of diabetes) manifested by symptoms of “edema”, “kidney consumption” and “turbid urine” (17,18). The “Pi Dan”, “Xiao Ke”, and “Xiao Dan” recorded in the ancient Chinese book “Huang Di Nei Jing” are similar to “the hidden stage of diabetes”, “the clinical stage of diabetes”, and “the complications stage of diabetes”, respectively. Hence, early diabetic nephropathy belongs to the “Xiao Dan” category (19,20). Chinese medicine proposes that the five internal organs (heart, liver, spleen, kidney, lung) are essential, and the essence of the five internal organs is stored in the kidney. The main pathogenesis of early diabetic nephropathy is the aggravation of nephron damage, obvious damage to the five internal organs, and turbid toxin retention, which leads to the decline of the nephron (21,22). A large number of studies have shown that the use of traditional Chinese medicine in the treatment of diabetic nephropathy can not only control the course of the disease and improve the clinical symptoms, but also has fewer side effects and a higher safety profile than Western medicine (23,24). Among them, the Yiqi Huoxue prescription demonstrates relatively good therapeutic effects in the treatment of diabetic nephropathy (25).
To systematically evaluate the therapeutic effects of Yiqi Huoxue prescription in the treatment of diabetic nephropathy, a total of 13 reports were included in this study, and meta-analysis was performed. The SCr level reflects the glomerular filtration rate of the patient, the BUN reflects the glomerular function, and the UAER is the gold standard index for the diagnosis of early diabetic nephropathy (26,27). This study found that Yiqi Huoxue prescription on the basis of conventional Western medicine treatment could significantly reduce the UAER, SCr, and BUN levels in patients with diabetic nephropathy. This suggested that the combination of Yiqi Huoxue prescription combined with Western medicine could improve the clinical symptoms and renal function of patients with diabetic nephropathy.
Also, FBG and HbA1c is the gold standard for assessing blood glucose control, while TG and serum TC are important indexes for assessing patients’ blood lipids and blood viscosity (28,29). The results of this study revealed that Yiqi Huoxue prescription on the basis of conventional Western medicine treatment could reduce the serum FBG, HbA1c, triglyceride, and serum TC levels in patients with diabetic nephropathy. This indicated that the combination of Yiqi Huoxue prescription combined with Western medicine could obviously control the blood glucose level and reduce the blood viscosity of patients with diabetic nephropathy. Furthermore, it was found that the total effectiveness rate of Yiqi Huoxue prescription for diabetic nephropathy based on conventional Western medicine treatment was higher than that of conventional Western medicine treatment alone. However, only 9 indicators were involved. In the follow-up, more data is needed to strengthen the findings of the meta-analysis, and explore the long-term therapeutic effects.
In summary, the use of Yiqi Huoxue prescription on the basis of conventional Western medicine demonstrated good therapeutic effects for patients with diabetic nephropathy, which has broad clinical applications. These results provide a reference for the application of Yiqi Huoxue prescription in the clinical treatment of diabetic nephropathy.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://dx.doi.org/10.21037/apm-21-1147
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apm-21-1147). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Palumbo C, Nicolaci N, La Manna AA, et al. Association between central diabetes insipidus and type 2 diabetes mellitus. Medicina (B Aires) 2018;78:127-30. [PubMed]
- Wada J, Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci (Lond) 2013;124:139-52. [Crossref] [PubMed]
- Ioannou K. Diabetic nephropathy: is it always there? Assumptions, weaknesses and pitfalls in the diagnosis. Hormones (Athens) 2017;16:351-61. [PubMed]
- Nagib AM, Elsayed Matter Y, et al. Diabetic Nephropathy Following Posttransplant Diabetes Mellitus. Exp Clin Transplant 2019;17:138-46. [Crossref] [PubMed]
- Wang Y, Zhao H, Wang Q, et al. Chinese Herbal Medicine in Ameliorating Diabetic Kidney Disease via Activating Autophagy. J Diabetes Res 2019;2019:9030893 [Crossref] [PubMed]
- Cao X, Gong X, Ma X. Diabetic Nephropathy versus Diabetic Retinopathy in a Chinese Population: A Retrospective Study. Med Sci Monit 2019;25:6446-53. [Crossref] [PubMed]
- Dou XL, Wu JQ, Han LL, et al. The therapeutic effects of Yiqihuoxue herb on the diabetic kidney disease. Shanxi Med J 2012;41:440-2.
- Gou BW, Ji YL, Huang MF. Clinical observation of Yiqi Yangyin Huoxue herbs for the treatment of 60 cases of early diabetic nephropathy. J Tradit Chin Med 2012;12:1032-50.
- Guo Q, Chen ZQ, Fang J, et al. Clinical efficacy of Yiqi Yangyin xiaozheng Tongluo Fang on patients with stage IV diabetic nephropathy. J Beijing Univ Tradit Chin Med 2016;39:779-82.
- Li W, Cao XL, Wu GF, et al. Clinical Observation on 46 Cases of Early Diabetic Nephropathy Treated with the Method of Invigorating Qi, Nourishing Yin and Activating Blood. Front Med 2012;2:8-9.
- Liu H, Zheng J, Li RH. Clinical efficacy of 'Spleen-kidney-care' Yiqi Huayu and Jiangzhuo traditional Chinese medicine for the treatment of patients with diabetic nephropathy. Exp Ther Med 2015;10:1096-102. [Crossref] [PubMed]
- Wang FL, Chen ZQ, Wang YH, et al. Clinical observation of trasting early diabetic nephropathy by Qi supplementing, Yin nourishing, blood stasis dispersing, collateral dredging recipe. Zhongguo Zhong Xi Yi Jie He Za Zhi 2012;32:35-8. [PubMed]
- Su XW. Clinical analysis of the effect of replenishing qi and promoting blood circulation on diabetic nephropathy. Journal of Clinical Medical Literature 2016;3:8638-9.
- Wang SJ. Yiqi Yangyin Huoxue method for the treatment of diabetic nephropathy: a clinical curative effect analysis. J Chin Med Sci 2013;98:98-9.
- Wang MQ, Zhang JZ. Clinical trial of telmisartan and Qishen Yiqi drops in the treatment of patients with early diabetic nephropathy. Chin J Clin Pharmacol 2018;34:2610-2.
- Wu CX, Chen XC. Clinical observation of Yiqi Yangyin Huoxue herbs for the treatment of elderly diabetic nephropathy: 26 cases. Shaanxi J Tradit Chin Med 2007;28:977-80.
- Zhang N, Gao YX. Therapy of replenishing qi, nourishing and activating blood circulation used to treat diabetic nephropathy for reducing urinary albumin excretion rate. J Beijing Univ Tradit Chin Med 2009;32:274-7.
- Zhu X. Clinical observation of Qi huoxue fang on treatment of 30 cases of diabetic nephropathy. China Medical Guide 2011;20:147-9.
- Zou LH, Zhang JH, Liu PF, et al. Clinical observation on Qidi Yiqi Yangying Huoxue recipe in treating diabetic nephropathy at stage III and IV. Zhongguo Zhong Xi Yi Jie He Za Zhi 2006;26:1023-6. [PubMed]
- Wang L, Wang YH, Zhang XH, et al. Effectiveness comparisons of traditional Chinese medicine on treating diabetic nephropathy proteinuria: A systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e17495 [Crossref] [PubMed]
- Lu Z, Zhong Y, Liu W, et al. The Efficacy and Mechanism of Chinese Herbal Medicine on Diabetic Kidney Disease. J Diabetes Res 2019;2019:2697672 [Crossref] [PubMed]
- Wang B, Lin L, Ni Q, et al. Chinese medicine for treating diabetic nephropathy. Chin J Integr Med 2011;17:794-800. [Crossref] [PubMed]
- Yang X, Hu C, Wang S, et al. Clinical efficacy and safety of Chinese herbal medicine for the treatment of patients with early diabetic nephropathy: A protocol for systematic review and meta-analysis. Medicine (Baltimore) 2020;99:e20678 [Crossref] [PubMed]
- Sun GD, Li CY, Cui WP, et al. Review of Herbal Traditional Chinese Medicine for the Treatment of Diabetic Nephropathy. J Diabetes Res 2016;2016:5749857 [Crossref] [PubMed]
- Wang FL, Wang YH, Han L, et al. Renoprotective Effect of Yiqi Yangyin Huayu Tongluo Formula against Diabetic Nephropathy in Diabetic Rats. Evid Based Complement Alternat Med 2018;2018:4276052 [Crossref] [PubMed]
- Kishore L, Kaur N, Singh R. Distinct Biomarkers for Early Diagnosis of Diabetic Nephropathy. Curr Diabetes Rev 2017;13:598-605. [Crossref] [PubMed]
- Yu JY, An XF, Liu JS, et al. Plasma sRAGE is not associated with urinary microalbumin excretion in type 2 diabetic nephropathy at the early stage. Diabetes Res Clin Pract 2010;87:157-60. [Crossref] [PubMed]
- Zhang Q, Zhao G, Yang N, et al. Fasting blood glucose levels in patients with different types of diseases. Prog Mol Biol Transl Sci 2019;162:277-92. [Crossref] [PubMed]
- Park HS, Lim JH, Kim MY, et al. Resveratrol increases AdipoR1 and AdipoR2 expression in type 2 diabetic nephropathy. J Transl Med 2016;14:176. [Crossref] [PubMed]
(English Language Editor: A. Kassem)









