
Obstructive sleep apnea is associated with postoperative dialysis in patients who underwent coronary artery bypass grafting
Introduction
Obstructive sleep apnea (OSA) is the most common type of sleep disordered breathing, with a prevalence of 4–32.9% in the general population (1). The incidence of OSA is significantly high in patients diagnosed with cardiovascular disease, and it has been recognized as one of the risk factors, including hypertension, heart failure, and coronary artery disease (CAD) (2,3). In addition, the incidence of OSA among patients with CAD is >50%, according to different studies (4). OSA may increase the risk of perioperative complications in patients undergoing noncardiac surgery (5). Many studies have shown that OSA is associated with a higher prevalence of complications after cardiac surgery, mainly including valve procedures (6,7). Recently, few studies have reported that OSA is associated with a higher incidence of complications after coronary artery bypass grafting (CABG), including atrial fibrillation, prolonged hospital stay, and higher incidence of acute kidney injury (8-11). However, studies on the relationship among OSA, renal function, and dialysis during the perioperative period are not fully understood for those who underwent CABG. Therefore, we mainly studied the effect of OSA on baseline renal function and the use of dialysis after CABG.
We present the current study in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-21-180).
Methods
Study population
This prospective epidemiologic study included consecutive patients diagnosed with CAD who were referred to Beijing Anzhen Hospital from June 2019 to June 2020. The inclusion criteria included patients whose age >18 years and diagnosis of CAD who underwent CABG. All study procedures were conducted in accordance with institutional guidelines. In addition, we excluded patients who had previously been diagnosed with OSA, those with severe disability, and those with chronic kidney disease who required dialysis before surgery. All patients underwent polysomnography (PSG) before surgery. In addition, all patients provided informed consent prior to enrollment. Approval from the Ethics Committee of Capital Medical University Affiliated Anzhen Hospital (No. 2020034X) was obtained before the start of the study, and each participant signed a written consent form. All studies were conducted in accordance with the ethical principles stated in the Declaration of Helsinki (as revised in 2013) in the present study.
Measurements of renal function and other parameters
Fasting blood samples were collected from all patients on the second day of admission. We calculate the glomerular filtration rate using the following equation: a×(serum creatinine/b)c×(0.993)age; a=144 for women or 141 for men; b=0.7 for women or 0.9 for men; c=−0.329 if the creatinine is less than 0.7 mg/dL or −1.209 if the creatinine is larger than 0.7 mg/dL for women; c=−0.411 if the creatinine is less than 0.7 mg/dL or −1.209 if the creatinine is larger than 0.7 mg/dL for men.
Assessment of postoperative outcomes
In the present study, postoperative outcomes during the perioperative period included acute kidney injury, worsening of heart failure, myocardial infarction, and hemodynamic instability. A previous study defined postoperative acute kidney injury as an increase in serum creatinine ≥0.3 mg/dL or ≥1.5-fold the baseline requiring renal replacement therapy (12). Cardiac complications included worsening of heart failure and myocardial infarction, which was indicated by troponin I or by new ST segment elevations (>0.1 mV) (13). The need of vasopressor or inotropes 48 h after CABG was indicative of hemodynamic instability caused by any reasons of (13).
Polysomnography (PSG)
PSG was performed in all patients in our study using standard techniques (Embletta; Embla, UK). OSA is defined as at least 10 s of air flow through the mouth and nose in response to unsynchronized chest and abdominal effort. Hypopnea was defined as a 50% reduction in oral-nasal airflow for 10 s and a 4% reduction in oxygen saturation. Apnea (whether central, obstructive, or mixed) was defined as airflow cessation for ≥10 s. The classification of OSA is based on the presence of thoracic movements. The apnea-hypopnea index (AHI) was calculated using the total sleep time as the denominator. AHI was calculated as the average number of apnea and hypopnea per hour of sleep (14). In our study, mild and moderate to severe OSA was defined as AHI ≥5 events/h and 15 events/h, respectively (15).
Statistical analysis
The results in the present study are shown as mean ± SD, median (IQR), or percentage, when appropriate. Nominal variables were compared using χ2 or Fisher’s exact tests. One-way analysis of variance was used to compare the differences among the three groups. Multivariate linear regression models were used to determine the estimated glomerular filtration rate (eGFR). In addition, univariate and multivariate logistic regression analyses were used to determine the risk factors associated with dialysis after CABG. Variables with P<0.10 on univariate analysis, age, sex, and body mass index were entered into a multivariate analysis. All reported probability values in the present study were two-tailed, and a P value <0.05 was considered statistically significant. The SPSS version 24.0 software (IBM) and Prism GraphPad 7.0 (GraphPad Software Inc., La Jolla, CA, USA) were used for calculation and illustration, respectively.
Results
Baseline characteristics of the study population
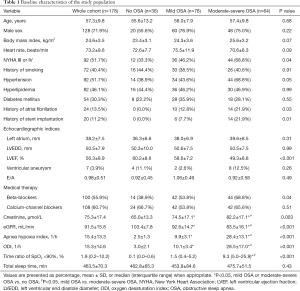
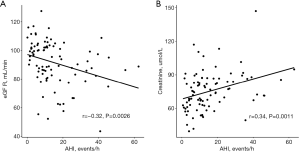
The baseline characteristics of the patients in our study are shown in Table 1, according to the severity of OSA. OSA was present in 142 (79.8%) patients, including 78 with mild OSA and 64 with moderate-to-severe OSA. There was no difference in age, body mass index, or male sex among the different groups. Compared with none and mild OSA patients, patients with moderate-to-severe OSA have a high prevalence of NYHA III or IV OSA. In addition, compared to patients without OSA, the prevalence of atrial fibrillation (AF) (0.0% vs. 12.8% vs. 21.9%, P=0.03) and stent implantation (0.0% vs. 7.7% vs. 21.9%, P=0.01) were higher in patients with OSA. Importantly, the level of creatinine was significantly increased with the severity of OSA, and the level of eGFR significantly decreased with OSA severity. Pearson correlation analysis revealed that the levels of eGFR (r=−0.32, P=0.0026) and creatinine (r=0.34, P=0.0011) were significantly affected by the severity of OSA, as reflected by AHI (Figure 1). Moreover, compared with patients without OSA, PSG parameters, including AHI, oxygen desaturation index, and time ratio of SpO2 <90%, were significantly higher in patients with OSA. However, no difference was found in the total sleep time among the different groups.
Full table
Baseline characteristics associated with eGFR
As shown in Table 2, in the multiple linear regression analysis, after adjustment for age, sex, body mass index, and other relevant variables from spearman analysis, only age (β=−0.29, P<0.001), male sex (β=0.17, P=0.02), creatinine level (β=−0.78, P<0.001), and AHI (β=−0.35, P<0.001) were associated with a decrease in eGFR (adjusted R2=0.376, P<0.001).

Full table
Perioperative data
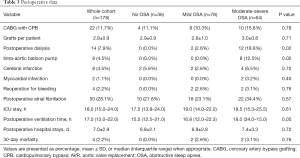
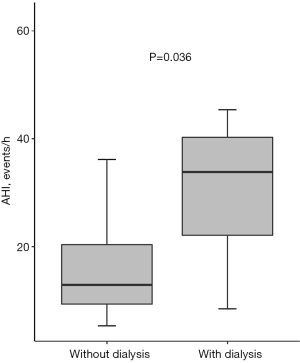
As shown in Table 3, the number of CABG with cardiopulmonary bypass and concomitant procedures did not differ among the groups. However, the number of dialysis (0.0% vs. 2.6% vs. 18.8%, P=0.02) and intra-aortic balloon pump (0.0% vs. 0.0% vs. 12.5%, P=0.02) were higher in patients with OSA. In addition, patients with OSA seemed to have the longer postoperative ventilation time [15.5 (12.5–21.0) vs. 16.8 (12.0–22.3) vs. 18.0 (34.0–15.0), P=0.05]. In addition, AHI was significantly higher in patients who underwent dialysis after CABG (Figure 2).
Full table
Predictor of postoperative dialysis after CABG
Finally, we further studied the factors that might be associated with a higher incidence of dialysis during the perioperative period. As shown in Table 4, the multivariate logistic regression model showed that a lower level of eGFR (OR =0.94, 95% CI, 0.89–0.99, P=0.02) and AHI (OR =1.07, 95% CI, 1.01–1.13, P=0.02) were independently associated with postoperative dialysis when adjusted for age, sex, body mass index, and other parameters.
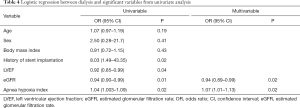
Full table
Discussion
In our study, we studied the impact of OSA on renal function and postoperative dialysis in patients who underwent CABG. The main findings of the present study are as follows: First, the prevalence of OSA was significantly higher in those who underwent CABG, accounting for 79.8% of patients. Second, the multiple linear regression model revealed that age, male sex, AHI, and creatinine were independently associated with a decrease in eGFR. Third, the levels of creatinine, eGFR, and AHI were independently associated with a higher rate of postoperative dialysis after CABG when adjusted for age, sex, and body mass index.
In the present study, the prevalence of OSA was approximately 79.8% in patients who underwent CABG, which is consistent with a previous study (10). OSA may affect the progression of arteriosclerosis and coronary plaque formation in CAD patients in several ways, including activation of the sympathetic nervous system, heart rate, blood pressure, and impaired vascular endothelial function (16). We found that the prevalence of hypertension, which is a risk factor for CAD patients, increased with the severity of OSA in our study, which is consistent with a previous study (17). Many studies have revealed that OSA is an independent risk factor for AF (18). Combined with our results, we believe that by increasing the risk of atrial fibrillation, OSA may contribute to the high NYHA class before surgery, which may represent a more severe disease state. Previous studies revealed that timely diagnosis and treatment of OSA in patients with CAD undergoing PCI can be regarded as a clinically relevant method of secondary prevention to decrease the risk of repeat revascularization (19). In our study, we also found that the proportion of patients with a history of stent implantation was significantly higher in patients with OSA, especially those with moderate-to-severe OSA. The mechanism by which OSA accelerates the development of CAD and requires further surgical treatment is unclear. Endothelial dysfunction triggered by oxidative stress and systemic inflammation, which are the results of intermittent hypoxemia occurring in OSA, may be the most important reason for the aggravation of CAD (20).
Previous studies revealed that pneumonia was the most frequent respiratory and infectious complication in patients who underwent CABG. Moreover, studies have shown that the incidence of AF is high, and OSA is an independent risk factor for postoperative AF after CABG (21,22). However, in the present study, we only found that the postoperative ventilation time was significantly longer than that in patients without OSA, and there was no difference in the prevalence of postoperative atrial fibrillation. The reason for this might be that the patients in our study were relatively young (10). Acute renal failure and hemodynamic instability were the most common complications in our study, reflected by a higher rate of postoperative intra-aortic balloon pump and dialysis. The elevated incidence of postoperative hemodynamic instability is probably due to the activation of the systemic nervous system, increased heart rate, and increased probability of cardiac arrhythmia due to OSA (23). These adverse factors lead to a decrease in blood pressure and require a large dose of vasoactive drugs to maintain hemodynamic stability.
Importantly, the strength of our study is that both the presence and severity of OSA were associated with a decrease in renal function and a higher rate of postoperative dialysis for patients undergoing CABG. Previous studies have reported that OSA might represent a novel risk factor and should be considered in the management of chronic kidney disease (24). In addition, another study revealed that sleep apnea is prevalent and associated with acute kidney injury after CABG (9). However, they did not study the relationship between dialysis, renal function, and OSA in these patients. Studies have shown that oxidative stress, chronic inflammation, activation of the renin-angiotensin system, and direct effects of hypoxia caused by OSA on the kidney might contribute to the impairment of renal function. In our study, there was no clinical manifestation of renal insufficiency in all patients before surgery, and the levels of creatinine and eGFR were within the normal range in most patients. However, compared to patients without OSA, both creatinine and eGFR were significantly changed in patients with OSA. Moreover, after adjustment for age, sex, and body mass index, OSA was also an independent risk factor for postoperative dialysis after CABG. All of these results indicate that we should pay more attention to these patients during the perioperative period, and OSA may need to be evaluated for patients who undergo CABG.
Previous studies have revealed that in a large prospective cohort study, OSA is an independent risk factor for adverse cardiovascular events in the general population, including sudden cardiac death (25). In addition, the severity of OSA is associated with cardiovascular risk, and effective treatment with continuous positive airway pressure can significantly reduce adverse cardiovascular outcomes in these patients (26). In addition, CPAP therapy can significantly decrease the risk of repeat revascularization after percutaneous coronary intervention (19). Importantly, recent studies have also shown that compliance with CPAP therapy is associated with a slower rate of progression of chronic kidney disease complicated by OSA (27). Therefore, we believe that timely treatment of these patients with CPAP may reduce perioperative complications, including postoperative hemodynamic instability and acute renal failure. It may also have a positive effect on long-term clinical results after CABG.
Limitations
As in other studies, the present study has some limitations. First, the sample size was relatively small, and this was a single-center study. Therefore, our results need to be confirmed by further large population studies, as well as multi-center combined clinical registry studies to confirm the effect of CPAP. Second, in our study, patients did not undergo Holter electrocardiographic monitoring, which is an important tool for determining any type of arrhythmia. Third, we only studied patients with OSA and did not analyze the impact of central sleep apnea on perioperative complications after CABG. Lastly, the clinical and prognostic significance of CPAP on renal function and perioperative complications after CABG remains unclear, and further studies are needed.
Conclusions
In conclusion, we found that OSA was associated with a decrease in renal function, and it was also an independent risk factor for postoperative dialysis in patients who underwent CABG. As the high incidence and negative prognostic implications of OSA are suggestive, screening for OSA in patients who undergo CABG is desirable and might provide a significant clinical benefit. Moreover, further studies are needed to evaluate the safety and clinical efficacy of OSA treatment in these patients, as well as their impact on long-term postoperative outcomes.
Acknowledgments
Funding: National Natural Science Foundation of China (Grant No. 81770371).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-21-180
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-21-180
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-21-180). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Approval from the Ethics Committee of Capital Medical University Affiliated Anzhen Hospital (No. 2020034X) was obtained before the start of the study, and each participant signed a written consent form. All studies were conducted in accordance with the ethical principles stated in the Declaration of Helsinki (as revised in 2013) in the present study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002;165:1217-39. [Crossref] [PubMed]
- Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet 2009;373:82-93. [Crossref] [PubMed]
- Baguet JP, Barone-Rochette G, Tamisier R, et al. Mechanisms of cardiac dysfunction in obstructive sleep apnea. Nat Rev Cardiol 2012;9:679-88. [Crossref] [PubMed]
- Prinz C, Bitter T, Piper C, et al. Sleep apnea is common in patients with coronary artery disease. Wiener medizinische Wochenschrift (1946) 2010;160:349-55. [Crossref] [PubMed]
- Memtsoudis S, Liu S, Ma Y, et al. Perioperative pulmonary outcomes in patients with sleep apnea after noncardiac surgery. A Anesth Analg 2011;112:113-21. [Crossref] [PubMed]
- Ding N, Ni BQ, Zhang XL, et al. Prevalence and risk factors of sleep disordered breathing in patients with rheumatic valvular heart disease. J Clin Sleep Med 2013;9:781-7. [Crossref] [PubMed]
- Ding N, Ni BQ, Wang H, et al. Obstructive Sleep Apnea Increases the Perioperative Risk of Cardiac Valve Replacement Surgery: A Prospective Single-Center Study. J Clin Sleep Med 2016;12:1331-7. [Crossref] [PubMed]
- Wong JK, Maxwell BG, Kushida CA, et al. Obstructive Sleep Apnea Is an Independent Predictor of Postoperative Atrial Fibrillation in Cardiac Surgery. J Cardiothorac Vasc Anesth 2015;29:1140-7. [Crossref] [PubMed]
- Kua J, Zhao LP, Kofidis T, et al. Sleep apnoea is a risk factor for acute kidney injury after coronary artery bypass grafting. Eur J Cardiothorac Surg 2016;49:1188-94. [Crossref] [PubMed]
- Rupprecht S, Schultze T, Nachtmann A, et al. Impact of sleep disordered breathing on short-term post-operative outcome after elective coronary artery bypass graft surgery: a prospective observational study. Eur Respir J 2017;49:1601486 [Crossref] [PubMed]
- Tafelmeier M, Weizenegger T, Ripfel S, et al. Postoperative complications after elective coronary artery bypass grafting surgery in patients with sleep-disordered breathing. Clin Res Cardiol 2018;107:1148-59. [Crossref] [PubMed]
- Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Critical Care 2007;11:R31. [Crossref] [PubMed]
- Mohammed AA, Agnihotri AK, van Kimmenade RRJ, et al. Prospective, Comprehensive Assessment of Cardiac Troponin T Testing After Coronary Artery Bypass Graft Surgery. Circulation 2009;120:843-50. [Crossref] [PubMed]
- Wang S, Cui H, Song C, et al. Obstructive sleep apnea is associated with nonsustained ventricular tachycardia in patients with hypertrophic obstructive cardiomyopathy. Heart Rhythm 2019;16:694-701. [Crossref] [PubMed]
- Wang S, Cui H, Zhu C, et al. Obstructive sleep apnea causes impairment of the carotid artery in patients with hypertrophic obstructive cardiomyopathy. Respir Med 2019;150:107-12. [Crossref] [PubMed]
- Arzt M, Hetzenecker A, Steiner S, et al. Sleep-Disordered Breathing and Coronary Artery Disease. Can J Cardiol 2015;31:909-17. [Crossref] [PubMed]
- Inami T, Seino Y, Otsuka T, et al. Links between sleep disordered breathing, coronary atherosclerotic burden, and cardiac biomarkers in patients with stable coronary artery disease. J Cardiol 2012;60:180-6. [Crossref] [PubMed]
- Kanagala R, Murali NS, Friedman PA, et al. Obstructive Sleep Apnea and the Recurrence of Atrial Fibrillation. Circulation 2003;107:2589-94. [Crossref] [PubMed]
- Wu X, Lv S, Yu X, et al. Treatment of OSA reduces the risk of repeat revascularization after percutaneous coronary intervention. Chest 2015;147:708-18. [Crossref] [PubMed]
- Wolf J, Lewicka J, Narkiewicz K. Obstructive sleep apnea: an update on mechanisms and cardiovascular consequences. Nutr Metab Cardiovasc Dis 2007;17:233-40. [Crossref] [PubMed]
- van Oosten EM, Hamilton A, Petsikas D, et al. Effect of preoperative obstructive sleep apnea on the frequency of atrial fibrillation after coronary artery bypass grafting. Am J Cardiol 2014;113:919-23. [Crossref] [PubMed]
- Wu CY, Wang SH, Shang YQ, et al. Incidence of atrial fibrillation after off-pump versus on-pump coronary artery bypass grafting: A meta-analysis of randomized clinical trials and propensity score matching trials. J Huazhong Univ Sci Technolog Med Sci 2017;37:956-64. [PubMed]
- Yumino D, Redolfi S, Ruttanaumpawan P, et al. Nocturnal Rostral Fluid Shift. Circulation 2010;121:1598-605. [Crossref] [PubMed]
- Voulgaris A, Marrone O, Bonsignore M, et al. Chronic kidney disease in patients with obstructive sleep apnea. A narrative review. Sleep Med Rev 2019;47:74-89. [Crossref] [PubMed]
- Lin CH, Lurie RC, Lyons OD. Sleep Apnea and Chronic Kidney Disease: A State-of-the-Art Review. Chest 2020;157:673-85. [Crossref] [PubMed]
- Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365:1046-53. [Crossref] [PubMed]
- Puckrin R, Iqbal S, Zidulka A, et al. Renoprotective effects of continuous positive airway pressure in chronic kidney disease patients with sleep apnea. Int Urol Nephrol 2015;47:1839-45. [Crossref] [PubMed]


