
Posterior wedge osteotomy assisted by O-arm navigation for treating ankylosing spondylitis with thoracolumbar fractures: an early clinical evaluation
Introduction
Ankylosing spondylitis (AS) is an autoimmune disease that affects the axial skeleton and is characterized by chronic complications of the spine and sacroiliac joints (1). The etiology of AS is complex and includes genetic, immune and microbial factors, and other factors (2). In 1973, HLA-B27 was identified as one genetic factor of AS (3). HLA-B7, HLA-B16, HLA-B35, HLAB38 and HLA-B39 are associated with HLA-B27 negative AS (4-7). IL-10 secreted by CD8+ T cells is increased in AS patients (8). Microbial infection could trigger the innate immune response and promote AS development (9). The disability rate of the disease is as high as 31% (10). Due to the characteristics of spinal fusion, osteoporosis, and spinal deformities of AS, these patients are more prone to fractures under low energy impact (11). Up to 21.7% of AS patients experience thoracolumbar fractures (12). As the thoracolumbar fractures of AS are unstable, the spinal cord and nerve roots are easily injured. It has been demonstrated that 67.2% of AS patients with thoracolumbar fractures have neurologic deficits, while secondary neurological deterioration occurs frequently (12). Due to its serious complications, the overall mortality rate of AS patients with thoracolumbar fractures within 3 months is 17.7%. At present, in the absence of surgical contraindications, surgical treatment should be performed promptly to correct the spinal deformity and retain the neurological functions (13).
There are a variety of surgical approaches for AS patients with thoracolumbar fractures, but the optimal surgical option has been controversial, because the surgery is difficult to perform and may be accompanied by some serious complications (14). The posterior approach is most commonly used relative to the anterior approach and anterior-posterior approach (15), due to it providing a strong holding force and reliable stability without involving extra operation time. Besides, osteotomy can be performed simultaneously through the posterior approach to address kyphosis deformity which commonly presents in the late stage of AS. Most importantly, the posterior approach is less traumatic (16). Therefore, posterior surgery is preferred by the majority of surgeons (17-20). In a previous study, they compared the treatment efficiency of long and short segment instrumentation on AS patients with no thoracolumbar fracture. They proved short segmental fixation is recommended for AS patients with bridging syndesmophytes (21). For patients with incomplete ossification of the anterior longitudinal ligament, the use of long segmental fixation is preferred. They didn’t report how to insert these screw effective and safety on AS patients with thoracolumbar fractures.
In order to ensure surgical safety and effectiveness, many surgeons use C-arm fluoroscopic imaging to assist during surgery (22). The traditional C-arm system only provides two-dimensional (2D) fluoroscopic images; the overlap of images can affect structural recognition, and cause iatrogenic intraoperative injuries such as spinal cord injury (SCI) or nerve root damage (23-25). Another imaging system, the O-arm navigation technique, provides real-time three-dimensional (3D) intraoperative imaging. During surgery with this technique, 3D images of the spine segments can be displayed simultaneously on the screen, furthering the surgeon’s understanding the situation of the diseased vertebral body (26,27). This advantage has led to O-arm navigation being used for the treatment of thoracolumbar fractures (28-30), and in recent years, many studies have shown that it is a safe and effective application in thoracolumbar surgery (31,32). In their review, Feng et al. concluded that O-arm navigation had significant advantages in accuracy over conventional C-arm fluoroscopy, although it had a comparatively lower efficiency outcome (33). However, there is currently no literature about O-arm navigation assisted AS thoracolumbar fractures surgery.
This study aimed to evaluate the effectiveness and safety of O-arm navigation assisted posterior wedge osteotomy in the treatment of AS patients with thoracolumbar fractures. We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/apm-21-1286).
Methods
Participant information
All patients were diagnosed with AS, according to the New York standard revised in 1984 (34). A total of 16 cases were included in this study, with 12 males and 4 females. The patients were from the department of orthopedic of Sun Yat-sen Memorial Hospital of Sun Yat-sen University from January 2012 to July 2015. The patients were followed up for 24-month after discharge. Participant ages ranged from 37 to 63 years old, and the average age was (42.5±5.4) years old. The AS history was 11 to 41 years, with an average of 23.5 years. The causes of injury included low fall (n=6), high fall (n=4), car accident (n=3), crush (n=2), and sprain (n=1). Magnetic resonance imaging (MRI) and X-ray were used to determine the fracture segment: T10 vertebral body (n=1), T11 vertebral body (n=3), T12 vertebral body (n=5), L1 vertebral body (n=4), and L2 vertebral body (n=3). All 16 participants had varying degrees of kyphosis. After completing relevant examinations and excluding surgical contraindications, “posterior wedge osteotomy assisted by O-arm navigation” was performed on all participants. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). This is a retrospective case series study of analyzing the clinical data of AS patients with thoracolumbar fracture. Thus, individual consent and ethic approval for this retrospective analysis was waived.
Intraoperative navigation system
An intraoperative navigation system (O-arm, Medtronic, Minneapolis, MN, USA) was used to provide standard fluoroscopy images or 3D computed tomography (CT) scans. After the surgical segment was located in the anteroposterior and lateral position, the O-arm navigation system fixed the dynamic reference frame at the adjacent spinous process. Then, with the help of the O-arm device, the intraoperative 3D reconstruction images (axial, coronal, and sagittal) were obtained and automatically transmitted to the navigation system.
Neurophysiological monitoring
An intraoperative neuromonitoring system (IOM16, Nicolet CR Endeavor, Conshohocken, PA, USA) was used to monitor neurological functions during surgery. Somatosensory evoked potentials (SEPs), motor evoked potentials (MEPs), and free-run electromyography (EMG) were monitored to prevent nerve injuries. It was deemed abnormal when one or more of the following conditions occurred during the operation: SEPs amplitude decline was greater than 50% and/or latency was extended by greater than 10%, MEP amplitude declined greater than 80%, and free-run EMG continued to display action potentials (35).
Surgical procedures
The participants were placed in a prone position under general anesthesia. The bending angle of the folding bed was adjusted to maintain a slight kyphosis and avoid further SCI. The O-arm was used to locate the fracture segment and the surgical area was routinely disinfected and draped. A longitudinal midline incision centered on the fractured vertebral body was made, subcutaneous tissue and deep fascia were cut sequentially. The paravertebral muscles were stripped to expose the fracture site. Then, the bilateral facet joint and transverse processes of the vertebral body were exposed. The upper and lower vertebral bodies were located and placed with register pins centered on the fractured body under the O-arm navigation. After confirming the position of register pins, the pedicle screw was implanted under the O-arm navigation. After a temporary rod was fixed on the pedicle screws, decompression laminectomy was performed, and the bilateral external walls of the fractured vertebral body were exposed with a periosteal stripper. A wedge osteotomy was performed through the pedicle at the site of the fractured vertebral body with a pedicle probe and drill to create a wedge resection space toward the upper segment of the damaged intervertebral disc. The sclerotic bone around the pseudarthrosis was also resected. The prepared bone block was inserted into the intervertebral space and the bone block was compacted. Finally, the wedge-shaped gap was closed and fracture reduction was performed at the same time. The pre-bent titanium rod was inserted and the nuts were tightened. After finishing the fixation, the O-arm was used to confirm that the decompression, reduction, and correction were satisfactory. Copious normal saline was used to rinse the wound. After strictly stopping the bleeding and checking the instrument, the drainage tube was positioned and the surgical incision was sutured.
Postoperative treatment and follow-up
The vital signs were detected by electrocardiogram (ECG) monitoring. Patients with surgical bleeding over 1,500 mL and surgery time over 3 h were treated with antibiotics for 1–2 days after surgery. Patients with SCI were treated with drugs to address dehydration and provide nerve nutrition. On the second day after the operation, the patients were encouraged to move the joints of their lower extremities. The drainage tube was removed within 48 h after the operation. The operation site was re-evaluated by X-ray. Participants wore a brace for protection within 3 months after discharge, returned to the outpatient department at 1, 3, and 6 months after the operation, and were re-examined via X-rays of the whole spine and pelvis. The neurological function after surgery was evaluated by American Spinal Injury Association (ASIA) scale. Detailed scoring criteria were shown in Table 1.
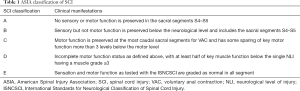
Full table
Pain evaluation
The visual analogue scale (VAS) was used to assess the degree of pain (36). The VAS is a 0 to 10 scale, where 0 means no pain and 10 means the heaviest pain. The decrease rate of VAS score ≥50% was considered pain relief.
Sagittal imaging parameters of the spine before and after surgery
Full spine and pelvic X-rays of the patient were taken in a standing position. Related parameters were measured on the lateral radiographs. The kyphosis Cobb angle was defined as the angle between the vertical lines of the tangent at the maximum inflection point of the kyphotic curve at both ends. The C7 plumb line (C7PL) was defined as the distance between the vertical line originating from the center of the C7 vertebral body and the posterior superior corner of S1. The chin-brow to vertical angle was defined as the angle between the line through the chin and brow and the vertical line of the ground.
Statistics and analysis
Complete participant information was collected, including the ASIA score (Table 2), VAS, Cobb angle of kyphosis, C7PL, chin-brow to vertical angle, and other parameters before and after surgery (Table 3). The quantitative data were presented as mean ± standard deviation. The software SPSS 17.0 (IBM Corp., Chicago, IL, USA) was used for statistical analysis. All data were analyzed using the t-test. Statistical significance was defined at P<0.05.
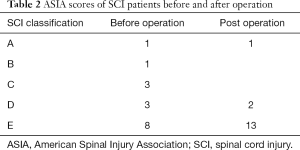
Full table

Full table
Results
The operative time consumption was 120–350 mins; the intraoperative blood loss volume was 200–800 mL. A single participant had a longer operation time and their bleeding volume reached 800 mL. Autologous blood transfusion was performed on this participant, and there were no other blood transfusions in the cohort. Intraoperative neuromonitoring was used in all 16 participants, and there were no abnormal waveforms in SEPs and MEPs. Surgical procedures such as pedicle screw placement had weak effects on nerves, and no spinal cord nerve injury occurred. There were no complications such as incision infection and deep vein thrombosis after operation. All participants underwent regular postoperative follow-up for 6–24 months. Participants with SCI had recovered to some extent (Table 2). The back pain of all participants was relieved after surgery (Table 3). Follow-up imaging data showed that 6 months after the operation, the fractured bone had healed, the spinal deformities of the participants were corrected to varying degrees, and the internal fixation position was good (Table 3).
Intraoperative neuro-electrophysiological monitoring
Intraoperative multimodal neuro-electrophysiological monitoring was used to monitor neurological injuries during surgeries. A total of 10 participants had waveform abnormalities during the operation. Of those 10 participants, eight had combined SCI before the operation. A single patient experienced SCI due to surgical operation. After intraoperative screw replacement and postoperative hormone application, the patient had no neurological damage postoperatively. One patient developed abnormal waveforms due to unreasonable muscle relaxants, which returned to normal after treatment. The remaining six participants without abnormal waveform showed no neurological dysfunction after surgery.
Typical cases
A 58-year-old male patient was diagnosed with AS 10 years prior. He had experienced severe chest and back pain without accompanying lower limb motor sensation dysfunction after a fall 1 month ago. His symptoms did not improve after 1 month of conservative treatment, and he was later admitted to our hospital. His condition was confirmed by X-ray, CT, and MRI (Figures 1-3) and was reported: AS with spinal fracture without SCI. After completing the other relevant examinations, “posterior wedge osteotomy assisted by O-arm navigation” was applied (Figure 4) and produced good treatment effect. Intraoperative neuromonitoring ensured that the surgery did not cause nerve injury (Figure 5). The specific imaging data after surgery are displayed in Figure 6.




Discussion
The condition of AS is a chronic progressive autoimmune inflammatory disease that mainly affects the spine and sacroiliac joints (37). The incidence of spinal fracture in AS patients is about 3.5 times that of normal unaffected people (38). Surgery is the first treatment choice for AS fracture. There have been extended debates over the best surgical approach for AS, including the anterior approach, posterior approach, and anterior-posterior approach. Some researchers have promoted the anterior-posterior approach (39), whereas others believe that posterior surgery alone can achieve excellent results (40). The anterior and posterior combined surgical approach was only used in 25% of patients as reported by Westerveld (12). Accumulated evidence suggests that posterior decompression can achieve more satisfactory clinical outcomes particularly for AS thoracolumbar fractures (15,40). Many techniques to reduce the risk of complications associated with posterior surgery have been demonstrated in various studies, and these techniques usually include C-arm fluoroscopy and O-arm navigation (22,31,32,41). Visibility of the upper thoracic vertebrae is poor due to shadow of the shoulder, scapula, and lung. In patients with osteoporosis and obesity, the image resolution of the C-arm is poor, making it difficult to see the outline of the bone. The O-arm navigation played a vital role in the success of the surgery. It was used to provide real-time multi-dimensional clear images without image overlap, which helped surgeons to achieve precise spinal cord decompression. With the help of O-arm navigation, even when kyphosis is very serious, the deformed spinal cord can be precisely decompressed (42). The O-arm navigation increases the safety and accuracy of the operation. The use of O-arm navigation provides compelling clinical results because it reduces the risk of complications and minimizes SCI.
We conducted a retrospective study which involved the corresponding clinical data of 16 AS patients with thoracolumbar fractures in order to evaluate the efficacy and safety of posterior wedge osteotomy assisted by O-arm navigation for treating AS with thoracolumbar fractures. The operative time consumption was 120–350 min and the intra-operative blood loss volume was 200–800 mL. All participants experienced relief of their back pain, and the neurological functions of 8 participants with SCI were recovered in varying degrees with the exception of 1 patient with severe SCI. The spinal deformities of participants were corrected to varying degrees. The fracture sites of 16 participants were all healed, and there was no loosening or detachment of internal fixation during the 2-year follow-up period. Meanwhile, no obvious complications occurred in any cases after operation which further exhibited the high efficacy and safety of posterior wedge osteotomy assisted by O-arm navigation in managing AS with thoracolumbar fractures.
It is common that AS patients are accompanied with osteoporosis. In AS, although the axial bone presents as a “bamboo like” spine and the intervertebral disc and ligament are extensively ossified (43), the vertebral body is osteoporotic. After fracture, it becomes easy for the vertebral cortex to be fragmented and embedded in the spinal canal, resulting in SCI. After thoracolumbar fracture, the biomechanics of the spine changes, and the stress concentration point is formed at the thoracolumbar junction (44), which is prone to nonunion and increases the risk of refracture. Fracture of AS is usually accompanied by dislocation, and because of spinal malunion, it is difficult to reset, affecting fracture healing. Due to these characteristics, the surgery needs to incorporate firm internal fixation and precise decompression. Surgery should be performed as soon as possible when the following surgical indications are present: (I) spinal fracture with over II° dislocation that is difficult to reduce or dislocate again; (II) MRI indicates surgery is required to remove the hematoma; (III) MRI confirms that a herniated disc is compressing the spinal cord. There are three division of AS fractures: shear fractures, stress fractures, and compression fractures (45). Shear fractures are common in the cervical spine (46), while stress fractures are more common in the thoracolumbar spine (47). Most AS thoracolumbar fractures compress the spinal cord and produce neurological symptoms. Surgery is essential to reduce compression of the spinal cord, optimize conditions for the recovery of spinal cord function, stabilize the spine, and avoid secondary injuries and complications from fractures. At present, the surgical approach of AS thoracolumbar fractures mainly includes three approaches, with each of the three having its own advantages and disadvantages. In 1959, Boucher (48) proposed the “posterior approach” for the treatment of AS spinal fractures, which could integrate the three-column structure of the spine to obtain multi-planar stability. This approach meets the biomechanical requirements of spinal fixation (49,50).
In our surgery, we selected the O-arm navigation assisted posterior wedge osteotomy for treating AS with thoracolumbar fractures. We performed long-segment fixation, and the fixed length was 2–3 segments above and below the fracture site. Assisted by O-arm navigation, precise osteotomy was performed on the pedicle part, and the osteotomy angle was controlled within the range where the spine biomechanics are stable. Finally, a wide range of effective osteotomy was performed to increase the contact surface of the fracture and further improve spinal stability.
The O-arm navigation played a vital role in the success of the surgery. It was used to provide real-time multi-dimensional clear images without image overlap, which helped surgeons to achieve precise spinal cord decompression. With the help of O-arm navigation, even when kyphosis is very serious, the deformed spinal cord can be precisely decompressed (42). The O-arm navigation increases the safety and accuracy of the operation. Posterior wedge osteotomy is applied to treat spine kyphosis, it could not be used to treat intervertebral disc herniation. Some type of intervertebral disc herniation could be treated by minimally invasive surgery that guided by O-arm navigation.
Patients with AS may develop spinal fusion, deformity, and rigidity in the later stages of the disease, which seriously affects motor function (44). However, for patients with AS fracture and kyphotic deformity, there is still controversy surrounding whether the deformity can be concurrently corrected during the fracture fixation surgery (51). Spinal deformity in AS patients is mainly manifested as sagittal imbalance. Jean and other researchers have found that compared with coronal balance, sagittal balance has a stronger correlation with health-related quality of life (52). Some academics believe that kyphosis is an important cause of low back pain and is also a major risk factor for spinal fractures (53). In this study, all participants had thoracolumbar kyphosis, and all were treated with osteotomy intraoperatively. The fracture sites of these participants mainly occurred in the kyphotic segment, which provides the right conditions for our osteotomy and correction. The question remained about whether correction is required when the fracture site is not in the kyphotic segment. Zhang et al. found that if the patient's general condition is good and the spinal fracture is handled smoothly, osteotomy can be considered at the same time (54); however, some patients have a poor general condition, complicated fractures, and severe spinal nerve injury, which lengthen the operation time. In such cases, fracture fixation is recommended first, and deformity correction should be carried out in the second stage.
However, this study had some shortcomings: except for two database-based studies, the number of AS patients with thoracolumbar fracture was around 10 (13-15,22,55-59). Although some studies have a longer time range, the number of patients in this category is small. The number of patients in this study was 16, which is still a small number, but slightly more than in previous studies. Later, the database should be used to conduct large sample studies, so as to better draw meaningful research conclusions. We did not quantitatively analyze the effect of O-arm navigation in this surgery, and the number of cases and follow-up time were not optimized. We will implement corrections and improvements in the next study.
Conclusions
Our retrospective study suggested that incorporating O-arm navigation into posterior thoracolumbar surgery, such as posterior wedge osteotomy, appeared to be effective and safe, since no obvious complications occurred in any cases postoperatively. All participants experienced back pain relief, and the neurological functions of eight participants with SCI were recovered in varying degrees. Therefore, O-arm navigation may play a significant role in posterior wedge osteotomy since it may enhance surgical safety and efficiency.
Acknowledgments
Funding: This study was supported by the Science and Technology Program of Guangzhou, China (201707010089), Medical Science and Technology Research Foundation of Guangdong Province, Guangzhou, China (A2021371), Funding of Basics and Application Basics of Guangzhou (202102020096), and Funding of Regenerative Medicine and Health Laboratory of Guangzhou, Guangdong (1102101201).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/apm-21-1286
Data Sharing Statement: Available at https://dx.doi.org/10.21037/apm-21-1286
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apm-21-1286). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). This is a retrospective case series study of analyzing the clinical data of AS patients with thoracolumbar fracture. Thus, individual consent and ethic approval for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Adams MA, Pollintine P, Tobias JH, et al. Intervertebral disc degeneration can predispose to anterior vertebral fractures in the thoracolumbar spine. J Bone Miner Res 2006;21:1409-16. [Crossref] [PubMed]
- Zhu W, He X, Cheng K, et al. Ankylosing spondylitis: etiology, pathogenesis, and treatments. Bone Res 2019;7:22. [Crossref] [PubMed]
- Brewerton DA, Hart FD, Nicholls A, et al. Ankylosing spondylitis and HL-A 27. Lancet 1973;1:904-7. [Crossref] [PubMed]
- Wei JC, Sung-Ching HW, Hsu YW, et al. Interaction between HLA-B60 and HLA-B27 as a Better Predictor of Ankylosing Spondylitis in a Taiwanese Population. PLoS One 2015;10:e0137189 [Crossref] [PubMed]
- Khan MA, Kushner I, Braun WE. B27-negative HLA-BW16 in ankylosing spondylitis. Lancet 1978;1:1370-1. [Crossref] [PubMed]
- Wagener P, Zeidler H, Eckert G, et al. Increased frequency of HLA-Bw62 and Bw35 CREG antigens in HLA-B27 negative ankylosing spondylitis. Z Rheumatol 1984;43:253-7. [PubMed]
- Yamaguchi A, Tsuchiya N, Mitsui H, et al. Association of HLA-B39 with HLA-B27-negative ankylosing spondylitis and pauciarticular juvenile rheumatoid arthritis in Japanese patients. Evidence for a role of the peptide-anchoring B pocket. Arthritis Rheum 1995;38:1672-7. [Crossref] [PubMed]
- Rudwaleit M, Siegert S, Yin Z, et al. Low T cell production of TNFalpha and IFNgamma in ankylosing spondylitis: its relation to HLA-B27 and influence of the TNF-308 gene polymorphism. Ann Rheum Dis 2001;60:36-42. [Crossref] [PubMed]
- Sieper J, Braun J, Kingsley GH. Report on the Fourth International Workshop on Reactive Arthritis. Arthritis Rheum 2000;43:720-34. [Crossref] [PubMed]
- Prince DS, McGuigan LE, McGirr EE. Working life and physical activity in ankylosing spondylitis pre and post anti-tumor necrosis factor-alpha therapy. Int J Rheum Dis 2014;17:165-72. [Crossref] [PubMed]
- Wade W, Saltzstein R, Maiman D. Spinal fractures complicating ankylosing spondylitis. Arch Phys Med Rehabil 1989;70:398-401. [PubMed]
- Westerveld LA, Verlaan JJ, Oner FC. Spinal fractures in patients with ankylosing spinal disorders: a systematic review of the literature on treatment, neurological status and complications. Eur Spine J 2009;18:145-56. [Crossref] [PubMed]
- Olerud C, Frost A, Bring J. Spinal fractures in patients with ankylosing spondylitis. Eur Spine J 1996;5:51-5. [Crossref] [PubMed]
- Puvanesarajah V, Cancienne JM, Shimer AL, et al. Complications after Fusion for Thoracolumbar Fractures in Patients with Ankylosing Spondylitis. Global Spine J 2017;7:28-32. [Crossref] [PubMed]
- Hitchon PW, From AM, Brenton MD, et al. Fractures of the thoracolumbar spine complicating ankylosing spondylitis. J Neurosurg 2002;97:218-22. [PubMed]
- Liu R, Sun L, Li CH, et al. Cause analysis of spinal surgery in ankylosing spondylitis. Beijing Da Xue Xue Bao Yi Xue Ban 2017;49:835-9. [PubMed]
- Caron T, Bransford R, Nguyen Q, et al. Spine fractures in patients with ankylosing spinal disorders. Spine (Phila Pa 1976) 2010;35:E458-64. [Crossref] [PubMed]
- Sedney CL, Daffner SD, Obafemi-Afolabi A, et al. A Comparison of Open and Percutaneous Techniques in the Operative Fixation of Spinal Fractures Associated with Ankylosing Spinal Disorders. Int J Spine Surg 2016;10:23. [Crossref] [PubMed]
- Fordham S, Lloyd G. Treatment of the injured patient with ankylosing spondylitis. Praxis (Bern 1994) 2010;99:437-9.
- Yeoh D, Moffatt T, Karmani S. Good outcomes of percutaneous fixation of spinal fractures in ankylosing spinal disorders. Injury 2014;45:1534-8. [Crossref] [PubMed]
- Qiao M, Qian BP, Zhao SZ, et al. Clinical and Radiographic Results After Posterior Wedge Osteotomy for Thoracolumbar Kyphosis Secondary to Ankylosing Spondylitis: Comparison of Long and Short Segment. World Neurosurg 2018;117:e475-82. [Crossref] [PubMed]
- Kurucan E, Bernstein DN, Mesfin A. Surgical management of spinal fractures in ankylosing spondylitis. J Spine Surg 2018;4:501-8. [Crossref] [PubMed]
- Arand M, Schempf M, Kinzl L, et al. Precision in standardized Iso-C-Arm based navigated boring of the proximal femur. Unfallchirurg 2001;104:1150-6. [Crossref] [PubMed]
- Nakagawa H, Kamimura M, Uchiyama S, et al. The accuracy and safety of image-guidance system using intraoperative fluoroscopic images: an in vitro feasibility study. J Clin Neurosci 2003;10:226-30. [Crossref] [PubMed]
- Rampersaud YR, Foley KT, Shen AC, et al. Radiation exposure to the spine surgeon during fluoroscopically assisted pedicle screw insertion. Spine (Phila Pa 1976) 2000;25:2637-45. [Crossref] [PubMed]
- Epstein NE. Commentary: Utility of the O-Arm in spinal surgery. Surg Neurol Int 2014;5:S517-9. [Crossref] [PubMed]
- Schils F. O-arm guided balloon kyphoplasty: preliminary experience of 16 consecutive patients. Acta Neurochir Suppl 2011;109:175-8. [Crossref] [PubMed]
- Ge RL, Yang P, Liu X, et al. Comparison of percutaneous pedicle screw placement under O-arm navigation with traditional percutaneous pedicle screw placement in patients with thoracolumbar fractures without neurological symptoms. Zhonghua Yi Xue Za Zhi 2020;100:3099-103. [PubMed]
- Lu J, Chen W, Liu H, et al. Does Pedicle Screw Fixation Assisted by O-Arm Navigation Perform Better Than Fluoroscopy-guided Technique in Thoracolumbar Fractures in Percutaneous Surgery?: A Retrospective Cohort Study. Clin Spine Surg 2020;33:247-53. [Crossref] [PubMed]
- Zhang Y, Liu H, He F, et al. Safety and efficacy of percutaneous kyphoplasty assisted with O-arm navigation for the treatment of osteoporotic vertebral compression fractures at T6 to T9 vertebrae. Int Orthop 2020;44:349-55. [Crossref] [PubMed]
- Shin MH, Hur JW, Ryu KS, et al. Prospective Comparison Study Between the Fluoroscopy-guided and Navigation Coupled With O-arm-guided Pedicle Screw Placement in the Thoracic and Lumbosacral Spines. J Spinal Disord Tech 2015;28:E347-51. [Crossref] [PubMed]
- Silbermann J, Riese F, Allam Y, et al. Computer tomography assessment of pedicle screw placement in lumbar and sacral spine: comparison between free-hand and O-arm based navigation techniques. Eur Spine J 2011;20:875-81. [Crossref] [PubMed]
- Feng W, Wang W, Chen S, et al. O-arm navigation versus C-arm guidance for pedicle screw placement in spine surgery: a systematic review and meta-analysis. Int Orthop 2020;44:919-26. [Crossref] [PubMed]
- van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361-8. [Crossref] [PubMed]
- Bhagat S, Durst A, Grover H, et al. An evaluation of multimodal spinal cord monitoring in scoliosis surgery: a single centre experience of 354 operations. Eur Spine J 2015;24:1399-407. [Crossref] [PubMed]
- Chen LH, Kao FC, Niu CC, et al. Surgical treatment of spinal pseudoarthrosis in ankylosing spondylitis. Chang Gung Med J 2005;28:621-8. [PubMed]
- Braun J, Sieper J. Ankylosing spondylitis. Lancet 2007;369:1379-90. [Crossref] [PubMed]
- Mansour M, Cheema GS, Naguwa SM, et al. Ankylosing spondylitis: a contemporary perspective on diagnosis and treatment. Semin Arthritis Rheum 2007;36:210-23. [Crossref] [PubMed]
- Altenbernd J, Bitu S, Lemburg S, et al. Vertebral fractures in patients with ankylosing spondylitis: a retrospective analysis of 66 patients. Rofo 2009;181:45-53. [Crossref] [PubMed]
- Sapkas G, Kateros K, Papadakis SA, et al. Surgical outcome after spinal fractures in patients with ankylosing spondylitis. BMC Musculoskelet Disord 2009;10:96. [Crossref] [PubMed]
- Patel K, Tajsic T, Budohoski KP, et al. Simultaneous navigated cervico-thoracic and thoraco-lumbar fixation. Eur Spine J 2018;27:318-22. [Crossref] [PubMed]
- Tabaraee E, Gibson AG, Karahalios DG, et al. Intraoperative cone beam-computed tomography with navigation (O-ARM) versus conventional fluoroscopy (C-ARM): a cadaveric study comparing accuracy, efficiency, and safety for spinal instrumentation. Spine (Phila Pa 1976) 2013;38:1953-8. [Crossref] [PubMed]
- Vosse D, Landewé R, van der Heijde D, et al. Ankylosing spondylitis and the risk of fracture: results from a large primary care-based nested case-control study. Ann Rheum Dis 2009;68:1839-42. [Crossref] [PubMed]
- Anderson JJ, Baron G, van der Heijde D, et al. Ankylosing spondylitis assessment group preliminary definition of short-term improvement in ankylosing spondylitis. Arthritis Rheum 2001;44:1876-86. [Crossref] [PubMed]
- Werner BC, Samartzis D, Shen FH. Spinal Fractures in Patients With Ankylosing Spondylitis: Etiology, Diagnosis, and Management. J Am Acad Orthop Surg 2016;24:241-9. [Crossref] [PubMed]
- Ticó N, Ramon S, Garcia-Ortun F, et al. Traumatic spinal cord injury complicating ankylosing spondylitis. Spinal Cord 1998;36:349-52. [Crossref] [PubMed]
- Chan FL, Ho EK, Fang D, et al. Spinal pseudarthrosis in ankylosing spondylitis. Acta Radiol 1987;28:383-8. [Crossref] [PubMed]
- BOUCHER HH. A method of spinal fusion. J Bone Joint Surg Br 1959;41-B:248-59. [Crossref] [PubMed]
- McLain RF. The biomechanics of long versus short fixation for thoracolumbar spine fractures. Spine (Phila Pa 1976) 2006;31:S70-9; discussion S104. [Crossref] [PubMed]
- Samartzis D, Anderson DG, Shen FH. Multiple and simultaneous spine fractures in ankylosing spondylitis: case report. Spine (Phila Pa 1976) 2005;30:E711-5. [Crossref] [PubMed]
- Chang KW, Tu MY, Huang HH, et al. Posterior correction and fixation without anterior fusion for pseudoarthrosis with kyphotic deformity in ankylosing spondylitis. Spine (Phila Pa 1976) 2006;31:E408-13. [Crossref] [PubMed]
- Jean L. Influence of age and sagittal balance of the spine on the value of the pelvic incidence. Eur Spine J 2014;23:1394-9. [Crossref] [PubMed]
- Sudo H, Abe Y, Kokabu T, et al. Correlation analysis between change in thoracic kyphosis and multilevel facetectomy and screw density in main thoracic adolescent idiopathic scoliosis surgery. Spine J 2016;16:1049-54. [Crossref] [PubMed]
- Zhang H, Zhou Z, Guo C, et al. Treatment of kyphosis in ankylosing spondylitis by osteotomy through the gap of a pathological fracture: a retrospective study. J Orthop Surg Res 2016;11:136. [Crossref] [PubMed]
- Zhang W, Zheng M. Operative strategy for different types of thoracolumbar stress fractures in ankylosing spondylitis. J Spinal Disord Tech 2014;27:423-30. [Crossref] [PubMed]
- Lu ML, Tsai TT, Lai PL, et al. A retrospective study of treating thoracolumbar spine fractures in ankylosing spondylitis. Eur J Orthop Surg Traumatol 2014;24:S117-23. [Crossref] [PubMed]
- An SB, Kim KN, Chin DK, et al. Surgical outcomes after traumatic vertebral fractures in patients with ankylosing spondylitis. J Korean Neurosurg Soc 2014;56:108-13. [Crossref] [PubMed]
- Tan T, Huang MS, Hunn MK, et al. Patients with ankylosing spondylitis suffering from AO Type B3 traumatic thoracolumbar fractures are associated with increased frailty and morbidity when compared with patients with diffuse idiopathic skeletal hyperostosis. J Spine Surg 2019;5:425-32. [Crossref] [PubMed]
- Mata-Gómez J, Gilete-Tejero IJ, Rico-Cotelo M, et al. Neurologically Asymptomatic Lumbar Traumatic Dislocation With Vascular Compression in a Patient With Ankylosing Spondylitis: Case Report. Int J Spine Surg 2021;14:S16-20. [Crossref] [PubMed]
(English Language Editor: J. Jones)




