
Systematic review and meta-analysis of the efficacy of N-acetylcysteine in the treatment of acute exacerbation of chronic obstructive pulmonary disease
Introduction
Chronic obstructive pulmonary disease (COPD) is a general term for chronic obstructive airway diseases, which are clinically characterized by airflow limitation (1). At present, the specific pathogenesis of the cytology and molecular biology of COPD has not been clear, and the harmful gases and particles inhaled by long-term smoking as well as patients with chronic inflammatory diseases in respiratory tract will have an impact on the clinical treatment of COPD (2). COPD is the third most common cause of mortality worldwide (3). In 2012, more than 3 million patients died of COPD, accounting for 6% of all deaths globally.
A survey of 20,245 adults in seven regions in China has found that the prevalence of COPD among people over 40 years old is as high as 8.2%. Patients with COPD develop acute exacerbations between 0.5 and 3.5 times per year (4). AECOPD has four main clinical manifestations as follows: (I) the number of coughs increases obviously, which affects night sleep; (II) the sputum in the acute exacerbation period will becomes yellow and sticky sputum, the phlegm is yellow, and the amount of expectoration increases considerably; (III) dyspnea increases markedly; and (IV) obvious swelling (5). AECOPD promotes an increased hospitalization rate, increases medical expenses, seriously affects the labor capacity and quality of life of patients, and can even lead to death (6). Rosa et al. [2018] (7) pointed out that long-term inhalation of smoke and harmful particles can cause AECOPD and aggravate the condition. Airflow limitation in patients with AECOPD progresses for over an extended period, the onset is generally gradual and occurs over decades, and is common in middle-aged and elderly patients (8).
The main pathogenesis of AECOPD is oxidative stress, and antioxidant therapy can inhibit the decline of lung function in patients. N-acetylcysteine (NAC) is an antioxidant mucus dissolving agent, which can not only dissolve sputum, but also inhibit the production of oxides and improve antioxidant level (9). In recent years, with the extensive clinical use of NAC, more and more research results have shown that NAC has good efficacy in the treatment of respiratory interstitial pulmonary fibrosis, acute lung injury, and chronic obstructive pulmonary disease. Ansari et al. [2019] (10) pointed out that NAC could significantly reduce the incidence of AECOPD. In China, Guidelines for the Diagnosis and Treatment of Chronic Obstructive Pulmonary Disease in the Acute Exacerbation Period in 2007 pointed out that NAC can not only reduce the incidence of AECOPD, but also significantly reduce the severity of AECOPD. Glutathione sulfur transferase (GSH-ST) has a variety of biological functions, including repairing membrane phospholipid damage caused by free radicals, inhibiting the occurrence of microsomal peroxidation, and determining the activity of GSH-ST, so as to reflect the state of oxidative stress (11).
However, long-term multi-center randomized controlled trials are needed to determine whether the use of NAC treatment can significantly improve the lung function of patients with AECOPD. Currently, there is a lack of large-scale multicenter randomized controlled trials in China. The innovation of this study was to include a small sample of cases to quantitatively explain the efficacy of NAC in the treatment of AECOPD, so as to confirm that NAC could significantly improve the lung function and clinical symptoms of AECOPD patients. Relevant studies regarding NAC treatment of AECOPD (as the experimental group) were collected in this study, and were evaluated using the Cochrane system. Meta-analysis was then performed in terms of the following outcome indicators: improvement rate, forced expiratory volume in the first second (FEV1), FEV1/forced vital capacity (FVC), GSH-ST activity, hydroxyl radical inhibition ability, and superoxide anion radical resistance ability. Through this, a reliable theoretical basis could be provided for the clinical treatment of AECOPD.
We present the following article in accordance with the PRISMA reporting checklist (available at https://dx.doi.org/10.21037/apm-21-1138).
Methods
Literature search
We performed an electronic literature search of the PubMed, Medline, Cochrane Library, Chinese Biomedical Literature Database, China National Knowledge Infrastructure (CNKI), Wanfang, Weipu, Google Scholar, and other databases from the date of initiation of the database to November 25, 2020. The Boolean logic and compound logic search methods were adopted to select the relevant documents. The databases were searched using a combination of the following search terms: “chronic obstructive pulmonary disease”, “acute exacerbation of chronic obstructive pulmonary disease”, and “NAC”. The quality of the literature was evaluated using RevMan 5.3 software (Cochrane, Northern Europe) provided by the Cochrane system. In each database, a joint search strategy of free words and subject words was adopted. After multiple searches to confirm the documents, a search engine was used to track the confirmed documents, and the latest research progress was obtained after contacting experts and researchers in the field.
Literature inclusion and exclusion criteria
The inclusion criteria were defined as follows: (I) literature related to NAC treatment of AECOPD; (II) randomized controlled trials; (III) studies with a pathological control analysis and an index comparison that was reliable within a 95% confidence interval (CI); (IV) studies written in Chinese and English; (V) literature that included diagnosis of AECOPD according to the guidelines for its diagnosis and treatment formulated in China; and (VI) studies published from the date of initiation of the database to November 25, 2020.
The exclusion criteria were as follows: (I) literature that was irrelevant to this study; (II) research that did not include a control group; (III) repeatedly published articles; (IV) non-randomized controlled trials; (V) literature reviews, literature abstracts, case reports, and animal experiments; (VI) complete data could not be obtained by contacting the original author; and (VII) studies published in languages other than Chinese or English.
Outcome indicators
Six outcome indicators were evaluated in this study, including improvement rate, FEV1, FEV1/FVC, GSH-ST activity, hydroxyl radical inhibition ability (U/mL), and superoxide anion radical resistance ability (U/L).
Data extraction
The data were extracted independently by two experts through a unified Microsoft Excel table, and three pre-experiments were performed prior to extraction. Disagreements were resolved through discussion and consensus, or a third expert would be invited to arbitrate. The following data were extracted and included in this study: title of the research, name of the first author, date/year of publication, name of the publisher, basic information of the research objects (such as average age, gender, treatment plan, and drug dosage), grouping and statistical methods of the experimental and control groups, and the source of the cases, sample size, and outcome indicators.
Bias risk assessment
Two researchers simultaneously conducted a risk of bias assessment. Disagreements were resolved through discussion, or a third expert would be invited to arbitrate. In this study, the Cochrane Collaboration was used as a tool for “bias risk assessment” of randomized controlled experiments. The evaluation criteria were as follows: random allocation method, blind method, allocation plan concealment, completeness of data results, and research results. Judgments of “low risk of bias”, “unclear”, and “high risk of bias” were made according to each of the aforementioned five aspects.
Quality evaluation
Two researchers simultaneously conducted a risk assessment of bias, and disagreements were resolved through discussion, or a third expert could be asked to arbitrate. The scoring standards of the Oxford scoring system (JADAD score) were as follows: (I) whether the random allocation method was used correctly; (II) whether the allocation was hidden; (III) whether the blind method was adopted; (IV) the number of patients lost to follow-up or dropped out (as well as the reasons); and (V) whether the research results were fully explained. Studies were scored between 0 and 5 points. Documents scored 1–2 points were considered low quality (i.e., these studies had a high risk bias), and documents scored 3–5 points were considered high quality (i.e. low risk bias).
Statistical methods
Stata SE12.0 software (Stata, China) was used for statistical analysis. The risk rate was applied to evaluate the rate of improvement of NAC in the treatment of AECOPD, and the mean difference was employed to assess the FEV1, FEV1/FVC, GSH-ST activity, the ability to inhibit hydroxyl radicals, and the ability to resist superoxide anion free radicals. Also, the RevMan 5.3 (Cochrane, Northern Europe) software bias risk assessment chart was adopted to evaluate the risk bias of the included documents. Each effect was represented by a 95% CI. When P>0.1 and I2<50%, the fixed effects model was used for meta-analysis, whereas when P<0.1 and I2>50%, the random effects model was employed.
Results
Search results and basic information of included documents
A total of 1,281 documents were initially obtained. Of these, 1,241 documents were eliminated by reading the abstracts and titles, 25 documents were eliminated after reading the full text, and 15 documents were finally included in the meta-analysis. The main reasons for the exclusion of documents included duplicate research subjects (645 documents), document type that was not a case-control analysis (287 documents), research objects without chronic obstructive pulmonary disease (307 documents), and research related information that could not be extracted (25 documents) (Figure 1).
Figure 2 shows the quality grading results, demonstrating that there were nine documents with a score of 3–5 points, and six documents with a score of 1–2 points. Of the 15 documents that satisfied the inclusion criteria, 12 were retrospective analyses, and three were randomized controlled trials. In total, 1,605 patients were included. The 15 included studies were small sample studies, with sample sizes ranging from 72 to 146. The number of cases, ages, medication methods, and dosages of all documents were counted, and the general data of the research objects are presented in Table 1.
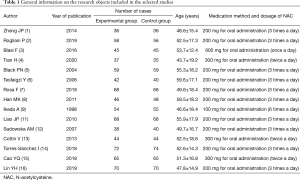
Full table
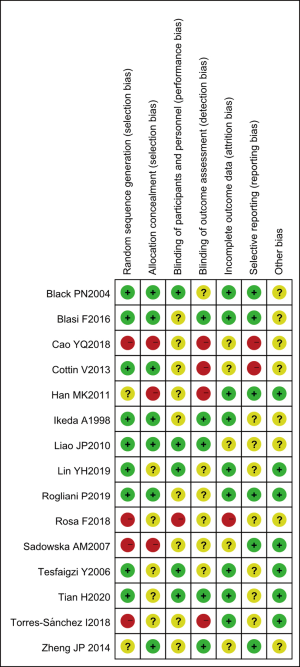
Risk of bias assessment
The results of multiple risk of bias assessments of the included literature (drawn using the Review Manager 5.3 software) are shown in Figures 3 and 4. Among the 15 studies included in this meta-analysis, two randomized controlled trials (12,13) described the correct random allocation method and also described the concealment of the allocation plan in detail. The measurement indicators in this study were laboratory indicators determined by a computer, and thus it could be considered that all documents were blinded correctly.
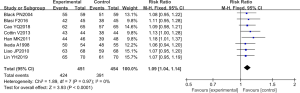
Comparison of improvement rate
The improvement rate was analyzed in eight randomized controlled experiments. In these studies, the total number of cases was 905, with 451 and 454 cases in the experimental and control groups, respectively. The overall heterogeneity test was performed [Chi2=1.89, df=7, I2=0% <50%, and P=0.97 (>0.01)], and the fixed effect model was adopted to analyze the whole. In almost all of these studies, the horizontal line of the 95% CI: crossed the right side of the invalid vertical line. The meta-analysis results revealed that the risk rate was 1.09 (95% CI: 1.04–1.14), and the difference was statistically significant (Z=3.93 and P<0.0001) (Figure 5).
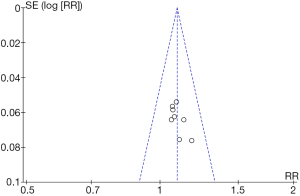
RevMan 5.3 was applied to obtain an improvement rate funnel chart (Figure 6). We found that the documents in some studies were basically symmetrical with the midline, suggesting that the research accuracy was high and that there was no publication bias.
Comparison of FEV1
FEV1 was analyzed in 10 randomized controlled experiments. The total number of cases was 1,049, with 525 cases in the experimental group and 524 cases in the control group. The overall heterogeneity test was carried out [Tau2=63.39, Chi2=118.66, df=9, I2=92% >50%, and P=0.88 (<0.0001)], and the random effect models were applied to analyze the whole. The horizontal line of the 95% CI: was to the right of the invalid vertical line in all of these studies. The meta-analysis results showed that the FEV1 of the experimental group was markedly higher than that of the control group; the mean difference was 30.63 (95% CI: 25.48–35.78), and the difference was statistically significant (Z=11.65 and P<0.0001) (Figure 7).
RevMan 5.3 was applied to obtain the funnel chart of FEV1 (Figure 8), which indicated that the circles in some studies were basically symmetrical with the midline, suggesting that the research accuracy was high and that there was no publication bias.
Comparison of FEV1/FVC
Six randomized controlled trials performed an analysis of the forced expiratory volume in the first second/forced vital capacity. These studies involved a total of 697 cases, with 347 cases in the experimental group, and 350 cases in the control group. The overall heterogeneity test was carried out (Tau2=60.03, Chi2=74.09, df=5, I2=93% >50%, and P<0.0001), and the random effect models were used for analysis on the whole. The horizontal line of the 95% CI: of all of these studies was to the right of the invalid vertical line. The meta-analysis results showed that the FEV1/FVC of the experimental group increased markedly compared with the control group; the mean difference was 30.42 (95% CI: 24.00–36.85), and the difference was statistically significant (Z=9.28 and P<0.0001) (Figure 9).
Figure 10 was the FEV1/FVC funnel chart obtained using RevMan 5.3. It revealed that the circles in some studies were basically symmetrical with the midline, showing that the research accuracy was high and that there was no publication bias.
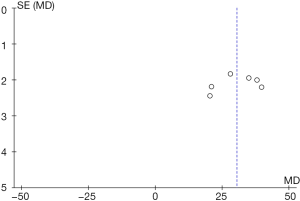
Comparison of GSH-ST activity
Six randomized controlled experiments analyzed GSH-ST activity, involving a total of 645 cases, with 323 cases in the experimental group and 322 cases in the control group. The overall heterogeneity test was performed (Tau2=4.12, Chi2=58.12, df=5, I2=91% >50%, and P<0.0001), with the analysis on the whole through the random effect models. In most of these studies, the horizontal line of the 95% CI: was on the right side of the invalid vertical line, whereas in a few of these studies, the horizontal line of the 95% CI: intersected the right side of the invalid vertical line. The meta-analysis results indicated that the GSH-ST activity of the experimental group was notably greater than that of the control group; the mean difference was 3.10 (95% CI: 1.38–4.82), and the difference was statistically significant (Z=3.63, P=0.0004) (Figure 11).
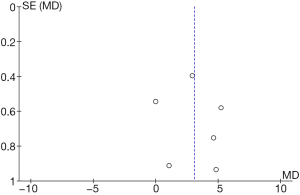
RevMan 5.3 was used to obtain a funnel chart of GSH-ST activity (Figure 12). It was found that the circles in some studies were basically symmetrical with the midline, signifying that the research accuracy was high and that there was no publication bias.
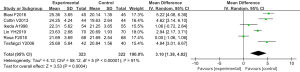
Comparison of the ability to inhibit hydroxyl radicals
Four randomized controlled experiments analyzed the ability to inhibit hydroxyl free radicals. In total, 451 cases were collected, including 224 cases in the experimental group and 227 cases in the control group. The overall heterogeneity test was carried out (Chi2=5.33, df=3, I2=44% <50%, and P=0.15 >0.01), using the fixed effect model to analyze the whole. The horizontal line of the 95% CI: of all of these studies was to the right of the invalid vertical line. The meta-analysis results showed that the ability of the experimental group to inhibit hydroxyl free radicals was higher than that of the control group; the mean difference was 77.52 (95% CI: 61.01–94.03), and the difference was statistically significant (Z=9.20 and P<0.0001) (Figure 13).

The funnel diagram of the ability to inhibit hydroxyl radicals (Figure 14) was obtained using RevMan 5.3, which indicated that that the circles in some studies were not symmetrical to the midline. This meant that the research accuracy was low and that the publication might be biased.
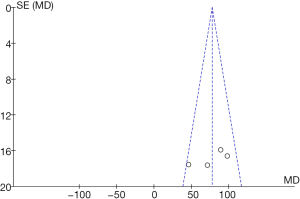
Comparison of superoxide anion radical resistance ability
Four randomized controlled experiments analyzed the superoxide anion radical resistance ability, involving a total of 451 cases, with 224 cases in the experimental group and 227 cases in the control group. The overall heterogeneity test was carried out [Chi2=4.59, df=3, I2=35% <50%, and P=0.20 (>0.01)], and the fixed effect model was adopted to analyze the whole. The horizontal line of the 95% CI: of all of these studies was to the right of the invalid vertical line. The meta-analysis results revealed that the superoxide anion radical resistance ability of the experimental group was greater than the ability of the control group; the mean difference was 47.75 (95% CI: 36.26–59.25), and the difference was statistically significant (Z=8.14 and P<0.0001) (Figure 15).
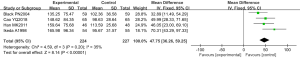
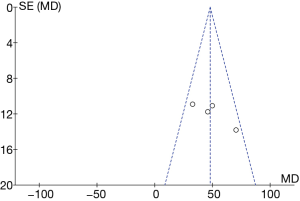
RevMan 5.3 was adopted to obtain a funnel diagram of the ability to resist superoxide anion free radicals (Figure 16). It indicated that the circles in some studies were not symmetrical to the midline, suggesting that the research accuracy was low and that the publication might be biased.
Discussion
NAC is an antioxidant that can improve the symptoms of patients with AECOPD by reducing oxidative stress (14). Through this meta-analysis, we found that after taking NAC, patients with AECOPD could sharply reduce their symptoms of cough, sputum, and dyspnea compared with the control group, with an improvement rate of 1.09 times, which was comparable to the research results of Cao et al. [2018] (15). This indicated that NAC could improve the clinical performance of patients.
NAC can improve the lung function and symptoms of patients, possibly by improving the oxidative stress-induced lung damage, inhibiting the thickening of the tracheal epithelium, and reducing the lung damage caused by the immune inflammatory response (16). In addition, NAC can dilute sputum to make it easier to expel quickly, hinder the growth of bacteria, and protect lung function. We performed a heterogeneity test of FEV1 (Tau2=63.39, Chi2=118.66, df=9, I2=92% >50%, P=0.88 <0.0001). The whole was analyzed by the random effect model, and the FEV1 was found to be markedly higher in the experimental group compared to the control group. The mean difference was 30.63 (95% CI: 25.48–35.78), and the difference was statistically significant (Z=11.65 and P<0.0001). However, Choi et al. [2019] (17) showed that there was no marked difference in the value of FEV1 during NAC treatment of AECOPD compared with the control group. In this study, NAC was taken orally while inhaling salbutamol, and the application of hormones could inhibit the occurrence of immune inflammatory reactions, which would have a certain impact on the results of NAC treatment of AECOPD.
The measured value of FVC is generally smaller than the actual value of vital capacity, which refers to the maximum volume of air that the patient exhales after inhaling (18). FEV1/FVC is a commonly used clinical indicator of lung function (19). Moreover, the FEV1/FVC of patients with chronic obstructive pulmonary diseases showed a downward trend. A heterogeneity test of FEV1/FVC was performed (Tau2=60.03, Chi2=74.09, df=5, I2=93% >50%, and P<0.0001). We found that the FEV1/FVC of the experimental group was markedly higher than that of the control group; the mean difference was 30.42 (95% CI: 24.00–36.85), and the difference was statistically significant (Z=9.28 and P<0.0001). This indicates that NAC treatment of AECOPD could considerably improve the lung function of patients.
GSH-ST is an anti-oxidative damage enzyme, and hydroxyl free radicals are the most active oxygen free radicals in patients. Superoxide anion is the condition for generating active oxygen. The inhibition ability of hydroxyl radical and superoxide anion radical is negatively correlated with the levels of hydroxyl radical and superoxide anion respectively, which can reflect the degree of promoting oxidation in vivo (20). The heterogeneity test of GSH-ST activity showed that Tau2=4.12, Chi2=58.12, df=5, I2=91% >50%, and P<0.0001. GSH-ST activity in the experimental group was markedly higher than that of the control group; the mean difference was 3.10 (95% CI: 1.38–4.82), and the difference was statistically significant (Z=3.63 and P=0.0004). Also, the heterogeneity test of the ability to inhibit hydroxyl free radicals showed that Chi2=5.33, df=3, I2=44% <50%, and P=0.15 >0.01, indicating that the ability of the experimental group to inhibit hydroxyl free radicals was higher than that of the control group (mean difference = 77.52, 95% CI: 61.01–94.03), and the difference was statistically significant (Z=9.20 and P<0.0001). The heterogeneity test of superoxide anion radical resistance ability showed that Chi2=4.59, df=3, I2=35% <50%, and P=0.20 >0.01), revealing that the superoxide anion radical resistance ability of the experimental group was higher than that of the control group (mean difference =47.75, the 95% CI: 36.26–59.25), and the difference was statistically significant (Z=8.14 and P<0.0001). Thus, it is suggested that NAC has obvious antioxidant capacity, which is beneficial to enhance the patient’s antioxidant capacity and promote their recovery.
Conclusions
Our meta-analysis confirmed that NAC can promote the symptom improvement rate of patients with AECOPD, improve lung function in FEV1 and FEV1/FVC, and enhance the body’s antioxidant capacity. The key limitation of this study is that the patient’s own condition, infection control, and nutritional support will have a certain impact on the improvement rate of lung function. In addition, the sample size of the literature included in this study was small. The sample size should be expanded in future randomized controlled trials to verify our findings. All in all, the results of this study can provide a reliable theoretical basis for the clinical treatment of AECOPD, so that patients can benefit from NAC treatment.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://dx.doi.org/10.21037/apm-21-1138
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apm-21-1138). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zheng JP, Wen FQ, Bai CX, et al. Twice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): a randomised, double-blind placebo-controlled trial. Lancet Respir Med 2014;2:187-94. [Crossref] [PubMed]
- Rogliani P, Matera MG, Page C, et al. Efficacy and safety profile of mucolytic/antioxidant agents in chronic obstructive pulmonary disease: a comparative analysis across erdosteine, carbocysteine, and N-acetylcysteine. Respir Res 2019;20:104. [Crossref] [PubMed]
- Blasi F, Page C, Rossolini GM, et al. The effect of N-acetylcysteine on biofilms: Implications for the treatment of respiratory tract infections. Respir Med 2016;117:190-7. [Crossref] [PubMed]
- Tian H, Zhou Y, Tang L, et al. High-dose N-acetylcysteine for long-term, regular treatment of early-stage chronic obstructive pulmonary disease (GOLD I-II): study protocol for a multicenter, double-blinded, parallel-group, randomized controlled trial in China. Trials 2020;21:780. [Crossref] [PubMed]
- Black PN, Morgan-Day A, McMillan TE, et al. Randomised, controlled trial of N-acetylcysteine for treatment of acute exacerbations of chronic obstructive pulmonary disease (ISRCTN21676344). BMC Pulm Med 2004;4:13. [Crossref] [PubMed]
- Tesfaigzi Y, Meek P, Lareau S. Exacerbations of chronic obstructive pulmonary disease and chronic mucus hypersecretion. Clin Appl Immunol Rev 2006;6:21-36. [Crossref] [PubMed]
- Rosa F, Bagnasco A, Ghirotto L, et al. Experiences of older people following an acute exacerbation of chronic obstructive pulmonary disease: A phenomenological study. J Clin Nurs 2018;27:e1110-9. [Crossref] [PubMed]
- Han MK, Martinez FJ. Pharmacotherapeutic approaches to preventing acute exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2011;8:356-62. [Crossref] [PubMed]
- Ikeda A, Nishimura K, Izumi T. Pharmacological treatment in acute exacerbations of chronic obstructive pulmonary disease. Drugs Aging 1998;12:129-37. [Crossref] [PubMed]
- Ansari SF, Memon M, Brohi N, et al. N-acetylcysteine in the Management of Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Cureus 2019;11:e6073 [Crossref] [PubMed]
- Liao JP, Chi CH, Li HC, et al. Effects of N-acetylcysteine on Clara cells in rats with cigarette smoke exposure. Chin Med J (Engl) 2010;123:412-7. [PubMed]
- Sadowska AM, Manuel-Y-Keenoy B, De Backer WA. Antioxidant and anti-inflammatory efficacy of NAC in the treatment of COPD: discordant in vitro and in vivo dose-effects: a review. Pulm Pharmacol Ther 2007;20:9-22. [Crossref] [PubMed]
- Cottin V. Clinical case: Combined pulmonary fibrosis and emphysema with pulmonary hypertension--clinical management. BMC Res Notes 2013;6:S2. [Crossref] [PubMed]
- Torres-Sánchez I, Valenza MC, Cebriá I. Effects of different physical therapy programs on perceived health status in acute exacerbation of chronic obstructive pulmonary disease patients: a randomized clinical trial. Disabil Rehabil 2018;40:2025-31. [Crossref] [PubMed]
- Cao YQ, Dong LX, Cao J. Pulmonary Embolism in Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Chin Med J (Engl) 2018;131:1732-7. [Crossref] [PubMed]
- Lin YH, Tsai CL, Tsao LI, et al. Acute exacerbations of chronic obstructive pulmonary disease (COPD) experiences among COPD patients with comorbid gastrooesophageal reflux disease. J Clin Nurs 2019;28:1925-35. [Crossref] [PubMed]
- Choi HS, Park YB, Shin KC, et al. Exacerbations of Chronic Obstructive Pulmonary Disease Tool to assess the efficacy of acute treatment. Int J Chron Obstruct Pulmon Dis 2019;14:471-8. [Crossref] [PubMed]
- Crisafulli E, Manco A, Torres A. How may we improve clinical outcomes for patients hospitalized with acute exacerbations of chronic obstructive pulmonary disease? A narrative review about possible therapeutic and preventive strategies. Expert Rev Respir Med 2020;14:493-500. [Crossref] [PubMed]
- Wu X, Shao C, Zhang L, et al. The effect of helium-oxygen-assisted mechanical ventilation on chronic obstructive pulmonary disease exacerbation: A systemic review and meta-analysis. Clin Respir J 2018;12:1219-27. [Crossref] [PubMed]
- Xiao W, Du LY, Mao B, et al. Endotype-driven prediction of acute exacerbations in chronic obstructive pulmonary disease (EndAECOPD): protocol for a prospective cohort study. BMJ Open 2019;9:e034592 [Crossref] [PubMed]
(English Language Editor: A. Kassem)








