
Prognostic evaluation model of diabetic nephropathy patients
Introduction
Diabetic nephropathy (DN), known as diabetic kidney disease (DKD) is the main cause of chronic kidney disease (CKD). About 17.4% of patients with DKD develop CKD, which eventually becomes end-stage kidney disease (ESRD) (1,2). In China, ESRD caused by DKD accounts for about 15% of the total number of ESRD patients (3,4). Few studies have identified DKD risk factors for progression into ESRD (5-7). Therefore, we explored the progression and the risk factors affecting prognosis of DN to provide a decision-making basis for its clinical treatment and established a line chart to predict the survival rate of type 2 DKD patients using a sample of 471 participants. We present the following article in accordance with the Tripod reporting checklist (available at https://dx.doi.org/10.21037/apm-21-1454).
Methods
General information
All participants were DKD patients treated in the Department of Nephrology at the Hangzhou Traditional Chinese Medicine Hospital. The inclusion criteria were as follows: (I) meet the diagnostic criteria of DN in the Chinese guidelines for the prevention and treatment of DN; (II) serum creatinine ≤265.2 µmol/L or creatinine clearance ≥30; (III) aged between 18 and 75 years; (IV) Exclusion of urinary tract infections, urinary tract tumors, nephritis, and other kidney diseases. The follow-up deadline for all participants was December 2020. In this study, end-stage nephropathy was used as the renal follow-up endpoint event. Data including age, gender, duration of diabetes (DD) were extracted, along with that concerning diabetic retinopathy eye-ground lesions (EL), body mass index (BMI), high blood pressure (HBP), creatinine (CR), and other clinical information. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Hangzhou Traditional Chinese Medicine Hospital Affiliated to Zhejiang Chinese Medical University (No.: 2021KY031) and informed consent was taken from all the patients.
Treatment
All participants were treated in accordance with the Chinese guidelines for the Prevention and treatment of DN: (I) controlling blood sugar, adjusting diet, improving lifestyle, oral hypoglycemic drugs, and insulin injection; (II) controlling blood pressure, according to the patient’s condition, select angiotensin-converting enzyme inhibitor (ACEI), angiotensin receptor blocker (ARB), and calcium antagonists; (III) limiting daily protein intake.
Observation indicators
Clinical data of type 2 DKD patients were retrospectively analyzed to extract the basic clinical participant characteristics, including gender, age, course of diabetes (years), hypertension and hypertension classification, CR, BMI, whether or not fundus lesions were present, and so on. Participants with hypertension were divided into 1–3 grades according to clinical diagnostic criteria, and those without hypertension were recorded as 0 grade. Participants were grouped into BMI of less than 18.5 (underweight), BMI of 18.5–24.9 (healthy), and BMI of above 25.0 (overweight). The patients with fundus lesions were classified as positive and those without were negative.
Statistical analysis
All statistical data are processed using R software and the related R packages (http://www.R-project.org/). The risk factors were determined by single factor Cox regression analysis and multi-factor Cox regression analysis, and the prognostic line map was established according to multi-factor Cox regression analysis. A Kaplan-Meier curve was drawn to analyze the survival of high and low risk groups, and the log-rank method was used for statistical testing. A receiver operating characteristic (ROC) curve was used to evaluate the prediction effect of the line diagram.
Results
General participant characteristics
This study included 241 patients, among them, 33 (7.0%) were lost to follow-up due to inaccessibility. Of remaining 438 type 2 diabetic kidney disease (DKD) patients, 305(69.6%) were male, 133 (30.4%) were female, the median age was 59 [23–73] years, median duration of diabetes was 93.1 [10 days–367 months], 301 (68.7%) participants had diabetic fundus lesions, 77 (17.6%) were without diabetic fundus lesions, 60 (13.7%) with undiagnosed diabetic fundus disease, 178 (40.6%) were overweight according to BMI, 253 (57.8%) were of normal weight, 7 (1.6%) participants were underweight, 78 (17.8%) were without hypertension, 105 (24.0%) participants had grade I hypertension, 168 (38.4%) had grade II hypertension, and 87 (19.9%) had grade III hypertension.
Patient survival and renal survival
A total of 95 (21.2%) deaths occurred during follow-up. Among them, 41 participants died from ESRD (9.36%), Other causes of death included tumors (n=5, 1.1%), cardiovascular and cerebrovascular events (n=27, 6.2%), accidental death (n=2 case, 0.2%), severe infection (n=5, 1.1%), and unknown causes (n=13, 3.1%). The median survival time of DKD participants was 63.1 months, and 201 cases (45.9%) entered the ESRD period during follow-up. The 3- and 5-year survival rates were 74.5% and 22.6%, respectively.
Risk factors influencing renal survival
The results of single factor Cox regression analysis showed that DD [hazard ratio (HR) 1.43–8.95, 95% confidence interval (CI): 1.268 to 1.629], EL (HR: 1.84–6.95, 95% CI: 1.189 to 2.866), BMI (HR: 1.25–0.95, 95% CI: 1.072 to 1.458), and HBP (HR: 1.22–2.95, 95% CI: 1.064 to 1.402) were prognostic risk factors in DKD patients and had adverse effects on prognosis (P<0.05) (Table 1).
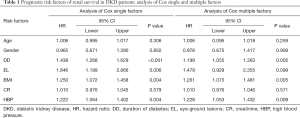
Full table
The results of multivariate Cox regression analysis showed that DD (HR: 1.199, 95% CI: 1.055 to 1.363), BMI (HR: 1.261, 95% CI: 1.073 to 1.451), and HBP (HR: 1.228, 95% CI: 1.053 to 1.432) were independent risk factors for renal survival and had adverse effects on prognosis (P<0.05) (Table 1).
Establishment and evaluation of a line chart
The prognostic evaluation chart was established according to the Cox multiple factor regression analysis, as shown in Figure 1. Age, diabetes course, fundus disease, BMI, and hypertension grade were all included in the scoring system.

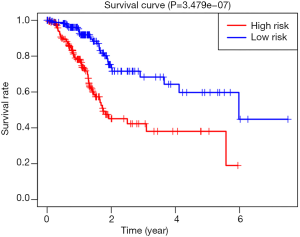
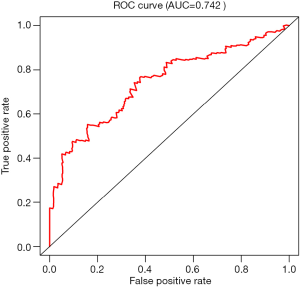
According to the line chart score, participants were divided into two groups, high risk and low risk, for survival analysis. As shown in Figure 2, the prognosis of participants in the low risk group was significantly better than that of those in the high risk group (P<0.05). The area under the curve (AUC) was 0.742, indicating that the line diagram prediction efficiency is good, as shown in Figure 3.
Discussion
The latest epidemiological report shows that outside of China, DKD prevalence is 28–40%, According to our literature, the prevalence of type 2 DN is 10–50% (8-10). The incidence of DN continues to increase annually (11,12). The clinical manifestations of DKD are obscure and complex; while concurrently, more serious complications accumulating in multiple sites throughout the body incur serious damage to the heart, brain, kidney, and other important organs (13-16). These complications accelerate the course of disease development to some extent (17). Eventually, DKD becomes ERSD, and it is also the primary cause of ERSD (18). The prognosis of most patients is grim; therefore, there is a great need to actively prevent the occurrence and development of DN, and improve the prognosis of such patients. The prognostic factors of DN are complex and variable.
Pathological changes, pathological types, urinary microalbumin, serum uric acid, and biological markers of renal tubular injury are all related to the prognosis of diabetic nephropathy. The pathological changes include glomerular lesions, vascular lesions, and tubular interstitial lesions. The degree of influence of these factors on the prognosis of diabetic nephropathy is not exact. The influence of pathological classification on its prognosis also needs to be further confirmed by large-scale clinical studies. And few studies have explored DKD prognostic risk factors (8,19). Unknown risk factors increase the difficulty of assessing disease development, and thus it is difficult to accurately guide clinical treatment.
In this study, a single factor Cox analysis was used to determine the DD, EL, BMI, and hypertension grade as risk factors for renal survival and prognosis in DKD patients, all of which have obvious adverse effects on prognosis. We used multifactorial Cox analysis to determine the DD, BMI, and hypertension classification, which is an independent risk factor for renal survival and prognosis in DKD patients. The above risk factors were shown to have obvious adverse effects on prognosis. We established a line map Cox predicting survival in DKD patients through multivariate analysis, including age, DD, EL, BMI, and hypertension grade in the scoring system. We calculated all participant risk scores according to the line chart score, and divided the participants into two groups: high risk and low risk. The survival analysis of the low risk group and high risk group was performed using Kaplan-Meier curve and log-rank tests. The results showed that survival of the low risk group was significantly better than that of the high risk group. To further confirm the efficacy of the line map prediction of DKD patient survival, we drew a ROC curve, and the AUC was greater than 0.6, which indicated that the line map prediction efficiency was good.
This study has some limitations. First of all, the sample size is too small, and the sample size needs to be further expanded. Second, there is a lack of independent external data to verify the model. Finally, the relevant indicators for establishing the model are limited to clinical variables and are relatively single, which may lead to biases in the results of the prediction model.
This study identified the prognostic risk factors of DKD patients, and established a line map to predict the prognosis survival rate of DKD patients according to the relevant clinical factors, which further confirmed the efficacy and clinical practicability of the line map. The prognosis of patients can be predicted accurately by this line diagram. It was shown to be a reliable model for predicting the prognosis of DN patients, and offered a basis for early identification of DN at risk of progression and the opportunity for clinical intervention to delay the progression of the disease.
Acknowledgments
Funding: This research is supported by the National Natural Science Foundation of China (81673913).
Footnote
Reporting Checklist: The authors have completed the Tripod reporting checklist. Available at https://dx.doi.org/10.21037/apm-21-1454
Data Sharing Statement: Available at https://dx.doi.org/10.21037/apm-21-1454
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apm-21-1454). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Hangzhou Traditional Chinese Medicine Hospital Affiliated to Zhejiang Chinese Medical University (No.: 2021KY031) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yu SM, Bonventre JV. Acute Kidney Injury and Progression of Diabetic Kidney Disease. Adv Chronic Kidney Dis 2018;25:166-80. [Crossref] [PubMed]
- Yang D, Livingston MJ, Liu Z, et al. Autophagy in diabetic kidney disease: regulation, pathological role and therapeutic potential. Cell Mol Life Sci 2018;75:669-88. [Crossref] [PubMed]
- Zhang L, Long J, Jiang W, et al. Trends in Chronic Kidney Disease in China. N Engl J Med 2016;375:905-6. [Crossref] [PubMed]
- Wen Y, Yan M, Zhang B, et al. Chinese medicine for diabetic kidney disease in China. Nephrology (Carlton) 2017;22:50-5. [Crossref] [PubMed]
- Yamanouchi M, Furuichi K, Hoshino J, et al. Nonproteinuric diabetic kidney disease. Clin Exp Nephrol 2020;24:573-81. [Crossref] [PubMed]
- Alicic RZ, Rooney MT, Tuttle KR. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin J Am Soc Nephrol 2017;12:2032-45. [Crossref] [PubMed]
- Tong L, Adler SG. Diabetic Kidney Disease. Clin J Am Soc Nephrol 2018;13:335-8. [Crossref] [PubMed]
- Tang SC, Yiu WH. Innate immunity in diabetic kidney disease. Nat Rev Nephrol 2020;16:206-22. [Crossref] [PubMed]
- Bonner R, Albajrami O, Hudspeth J, et al. Diabetic Kidney Disease. Prim Care 2020;47:645-59. [Crossref] [PubMed]
- Yang S, Zhang J, Feng C, et al. MTHFR 677T variant contributes to diabetic nephropathy risk in Caucasian individuals with type 2 diabetes: a meta-analysis. Metabolism 2013;62:586-94. [Crossref] [PubMed]
- Anders HJ, Huber TB, Isermann B, et al. CKD in diabetes: diabetic kidney disease versus nondiabetic kidney disease. Nat Rev Nephrol 2018;14:361-77. [Crossref] [PubMed]
- Pérez-Morales RE, Del PM, Valdivielso JM, et al. Inflammation in Diabetic Kidney Disease. Nephron 2019;143:12-6. [Crossref] [PubMed]
- Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care 2014;37:2864-83. [Crossref] [PubMed]
- Colhoun HM, Marcovecchio ML. Biomarkers of diabetic kidney disease. Diabetologia 2018;61:996-1011. [Crossref] [PubMed]
- Chen C, Wang C, Hu C, et al. Normoalbuminuric diabetic kidney disease. Front Med 2017;11:310-8. [Crossref] [PubMed]
- Reidy K, Kang HM, Hostetter T, et al. Molecular mechanisms of diabetic kidney disease. J Clin Invest 2014;124:2333-40. [Crossref] [PubMed]
- Lin YC, Chang YH, Yang SY, et al. Update of pathophysiology and management of diabetic kidney disease. J Formos Med Assoc 2018;117:662-75. [Crossref] [PubMed]
- Thomas MC, Brownlee M, Susztak K, et al. Diabetic kidney disease. Nat Rev Dis Primers 2015;1:15018. [Crossref] [PubMed]
- Forbes JM, Thorburn DR. Mitochondrial dysfunction in diabetic kidney disease. Nat Rev Nephrol 2018;14:291-312. [Crossref] [PubMed]
(English Language Editor: J. Jones)


