
Efficacy and safety of traditional Chinese medicine rehabilitation program in the treatment of knee osteoarthritis: a randomized controlled trial protocol
Introduction
Knee osteoarthritis (KOA) is a common joint disease worldwide, and the main cause of pain and disability in the elderly. In the past three decades, the prevalence of KOA has increased significantly in China (1). It has been reported that, among people older than 45 years, those with arthritis have a 2.4-fold increased risk of ≥2 falls and a 2.5-fold increased risk of fall injuries compared with those without arthritis (2). Due to increased aging and rates of obesity of the global population, the number of patients with KOA is expected to increase (3). The burden of patients with KOA is also expected to increase, and therefore, to improve the management of KOA, effective and safe interventions should be developed to reduce pain and improve range of motion, functionality, and quality of life.
Traditional Chinese medicine (TCM) has been accepted as a complementary and alternative therapy for KOA in both Asian and Western countries, most likely due to its effects on pain, depression, loss of function, and mobility (4-7). TCM is currently recommended for the non-surgical management of KOA (8). In the proposed randomized controlled study, we aim to evaluate the efficacy and safety of a TCM rehabilitation therapy in the treatment of KOA. We present the following article in accordance with the SPIRIT reporting checklist (available at https://dx.doi.org/10.21037/apm-21-1179).
Methods
Study design
The protocol of this multicenter, randomized, double-blind, parallel group, placebo-controlled trial will follow the SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) 2013 Statement (9).
Participant selection
The flow chart of this proposed study is showed in Figure 1. The inclusion criteria will be set as follows: (I) patients who meet the diagnostic criteria of KOA, according to the Diagnosis and Treatment of Osteoarthritis guidelines (1); (II) radiological diagnostic grade II or III, in accordance with Kellgren-Lawrence (K-L) criteria; (III) age between 50–80 years; and (IV) voluntary participation and provision of a signed informed consent form. The exclusion criteria will be set as follows: (I) patients with complications affecting the knee joint, such as psoriasis, syphilitic neuropathy, Charcot’s arthropathy, brown yellow disease, metabolic osteopathy, acute trauma; (II) patients with secondary or traumatic KOA; (III) those participating in other clinical trials that may influence the evaluation of the results of this study; (IV) those receiving intra-articular steroid injection or joint replacement in the past 3 months, intra-articular hyaluronic acid injection in the past 6 months, or total knee arthroplasty in the past year; (V) those unable to walk independently, and rely on assistive devices, such as a walking stick; and (VI) those who cannot cooperate with evaluation, treatment, and testing for other reasons.
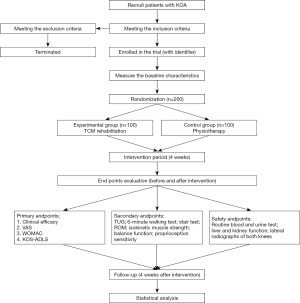
- Patients who cannot complete the trial due to complications or serious adverse events, or those who do not receive the required treatment and could not participate in the outcome assessment due to personal reasons will be suspended. Patients who could not provide informed consent and those who fail to complete the treatment or exercise procedure stipulated in the trial program and fail to follow up or with unclear curative effect will be regarded as withdrawal. Reasons for withdrawal will be clearly recorded, and the outcome indicators will be recorded and evaluated in detail. Patients with serious adverse events will be reported to the Ethics Committee of the Rehabilitation Hospital Affiliated to Fujian University of Traditional Chinese Medicine. These patients will be followed up to complete the evaluation projects.reduce the burden on the knee joint; (ii) avoid sitting and standing for a long time, and pay attention to the walking posture; (iii) wear soft flat shoes with thick and elastic soles to reduce the impact on the knee joint; (iv) perform warm-up activities during physical exercise and stretch your knee joints gently; and (v) keep warm and wear knee pads if necessary to prevent cold.
- Maintain normal living habits: (i) keep knees warm; (ii) avoid overweight and pressure on the knee; (iii) correct posture to avoid pressure on knee joint; (iv) when lifting heavy objects, use both hands to avoid excessive pressure on 1 side of the joint.
Experimental group
In addition to basic treatment, each patient in the experimental group will be treated with a TCM rehabilitation program for KOA. The treatment cycle will be 4 weeks, and patients will be treated 3 times a week, which is based on the clinical experience of TCM clinicians. The specific methods of the TCM rehabilitation program for the treatment of KOA are as follows:
- Acupuncture: acupoints on the affected side, such as calf nose, inner knee eye, Liangqiu, Xuehai, Weizhong, Zusanli, Yanglingquan, Yinlingquan, and Ashi, will be taken, and a filiform needle (0.3 mm × 40 mm) will be used. Patients will either sit or lay down. After local routine disinfection of each acupoint, the needle will be injected according to the characteristics of the acupoint, and the needle will be injected into the skin for 10–25 mm. Tonifying and relieving methods will be performed. Keep the needle still for 30 min after getting Qi.
- Massage manipulation: patients will be in the supine position. The first step is to relax the thigh quadriceps, and then to press kneading Fengshi, Fu Rabbit, Crane Ding, Xuehai and other acupoints. The second step is the kneading and plucking method to operate the patellar ligament and the medial and lateral collateral ligament, focusing on the inner and outer knee, Liangqiu, crane top, Zusanli, Yanglingquan, Xuehai, and other acupoints, and lift the patella. The third step is to use the rolling method to relax the muscles of the back of the thigh, popliteal fossa, and calf, and knead the Weizhong and Chengshan acupoints. Flexing the hip and knees, the doctor holds the patella on the affected side with 1hand, and holds the distal end of the leg with the other hand, and continues the knee flexion and shaking method, and cooperates with some knee passive actions such as rotation, flexion and extension and other passive movements. In the final step, the doctor rubs the affected knee using heat transmission. The treatment time of each knee will be 15 min.
- The skill of pile and sword in South Shaolin Station: this is mainly composed of three parts: body adjustment, breath adjustment, and heart adjustment.
- Key points of body adjustment:
- Preparatory type: this is based on the natural pile, and the detail is shown in the operation points of the natural pile.
- The center of gravity shifts to the right, the left foot crosses half a step to the left, the feet are parallel, and the feet are shoulder width apart.
- Bend knees and squat down into a horse-walking pile (divided into high, middle, and low positions according to the angle of knee bending).
- The knees naturally face outward, and the front edge of the patella does not exceed the toes of the feet vertically, so the knees and toes are in a straight line.
- As the knees are bent, arms are slowly raised straight ahead, while the palms naturally turn into sword fingers (index finger and middle finger close and straight like a sword, other fingers buckle slightly) to level with the shoulder, palm down, and the arms level with the shoulder, forming a horizontal line.
- The upper body is upright, the lower abdomen is slightly retracted, the tail is gently lifted, the chest and back are straight, the head and neck are straight, and the lower jaw is retracted, so that the midpoint of Baihui acupoint, perineal acupoint, and heel line are in a straight line.
- Look straight ahead, and the eyes are slightly closed. The whole body is relaxed and unremitting.
- Key points of breath adjustment:
- At the beginning of the training, the above operations can be carried out at the same time, using abdominal breathing, mainly cis-abdominal breathing, and exercise for 1 month.
- After 1 month of training, the exercise can be gradually transited to reverse abdominal breathing. The training of abdominal breathing can focus on exhalation and ignore the inhalation, with the mind leading the inner air into the abdomen.
- Key points of heart adjustment:
- Imagine an air mass in the abdomen, from small to large, from weak to strong, and then think that this air mass follows the foot Sanyin meridian, down to the foot Yongquan acupoint, and falls to the ground to take root. After that, he led the idea back to Dantian, making it from big to small, from strong to weak, pervading the whole body, nourishing the body and mind.
- Key points of body adjustment:
- Key points of three-adjustment-in-one
- After posing, with the induction of ideas, promote the warm air mass of Dantian to cooperate with abdominal breathing. When exhaling, the hot air mass diffuses downward along the foot Sanyin meridian on the inner side of the lower limb, and reaches the heart spring of the foot. When inhaling, the hot gas of the Dantian is introverted, and the gas at the tip of the finger is recovered to the chest along the hand Sanyin meridian. The air is introduced into the abdomen of pedestrians under any pulse, belonging to Dantian, and between one breath and one breath, the body, mind and breath are connected, and the three are integrated into one.
- Exercise frequency and intensity: exercise 4 times a day, 3–5 minutes each time, with an interval of 5 minutes each time, and exercise continuously for 4 weeks.
Control group
In addition to the basic treatment, follow the physiotherapy recommended by the International Osteoarthritis Research Association knee joint treatment guidelines (including strength exercise, aerobic exercise, balance training, neuromuscular training, physical and mental exercise, etc.) (10). The physiotherapy program will be completed under the one-to-one guidance of experienced therapists and trained for 40 minutes three times a week for 4 weeks:
- Improve the range of motion of knee joint flexion and extension: after the therapist loosened the fascia, loosened the patellofemoral joint and stretched the anterior thigh muscles, the supine position drooping leg exercise, 5min/group, 3 groups/time, twice a day. Then sit on the bed and put your hands around your ankles so that the ankles are slowly close to the buttocks. Then practice leg hook in prone position, 2 min/group, 3 groups/time, twice a day. Sitting position and knee extension: starting from 6 weeks after operation, the foot pad is high and a weight of 0.5 kg is added above the knee joint. Completely relax the muscles, maintain the 5 min group, 3 groups at a time, once a day, and begin to practice after 3 hours of leg bending.
- Neuromuscular re-education of quadriceps femoris: isometric contraction of quadriceps femoris can be performed with low-frequency electrical stimulation.
- Hip joint straight legs, straight legs raised to the heel out of bed 15 cm, held to 3 minutes, slowly down, 5 times each group, 2 groups a day, 60 seconds rest between groups until fatigue.
- Closed-chain movement of knee joint: squat in a small range (less than 90°); step on a bicycle in a high seat (resistance); squat against the wall when the heel is tolerable.
- Reduce swelling and relieve pain: under the premise of removing thrombus by color Doppler ultrasound of lower extremities, air pressure treatment for 20 minutes before treatment, cold compress of ice-water mixture after treatment, temperature control of 10–15 °C, cold compress for 10–15 minutes.
- Note: (i) the pain existing in the functional exercise is inevitable, and if it can fade to the original level within half an hour after the exercise stops, it will not cause damage to the tissue and should be properly tolerated; (ii) muscle strength exercises should be concentrated until the muscles feel sore and tired, and then proceed to the next group after full rest, the number of exercises, time and load depend on their own conditions, and the healthy side should be trained at the same time.
Observation time
The treatment cycle will be 4 weeks, and the curative effect will be evaluated and the indexes will be detected before and after treatment. Measures such as maintaining good communications with patients, and ensuring social and family supervision and support will be applied to increase the compliance of patients. The evaluation physician will not participate in the treatment and not know the case grouping.
Follow-up
Train the follow-up staff to learn the main contents, matters needing attention, language and language in the follow-up, and determine the consistency. The follow-up registration book and follow-up contact card shall be established by the evaluators, including the patient’s name, sex, age, occupation, telephone number, education level, illness, follow-up content and guidance measures, etc., and inform the patient or their family members at the same time. The evaluator will conduct telephone or outpatient follow-up, and record the evaluator’s name, telephone number, follow-up time or outpatient reexamination time on the follow-up card. The evaluators check the situation of the expired follow-up staff every day, and follow up the expired patients.
Follow-up time
Patients will be followed up within 1 month after the treatment.
Follow-up content
(I) Inquire about the dosage, frequency, time and frequency of treatment after treatment, (II) inquire about the symptoms and the specific time and frequency of recurrence, and to evaluate WOMAC score, VAS score, KOS-ADLS score and so on.
Follow-up method
Patients will be followed through telephone or outpatient.
Baseline characteristics
General items will include clinical trial institution, subject name, trial start date, residential address, contact number.
Biological characteristics
Demographic data
Demographic data will include sex, age, height, weight, nationality and so on.
Vital signs
Vital signs will include heart rate, blood pressure, abnormal symptoms and signs, if there are abnormal or special circumstances, record at any time.
Efficacy end point
Primary end points
Clinical efficacy outcomes
The clinical efficacy will be evaluated with the total score of the WOMAC rating scale as the standard, with reference to the Nimodipine method, that is, the index improvement rate = [(pre-treatment symptom score-post-treatment symptom score)/pre-treatment symptom score] ×100%. After recovery, the total score of WOMAC scale decreased by >75%; the total score of WOMAC scale decreased by 50–75%; effectively, the total score of WOMAC scale decreased by 30–50%; ineffective, the total score of WOMAC scale decreased by <30%.
Total effective rate = (cure + effective + effective)/total number of cases ×100%.
Visual analogue scale (VAS)
Use a long line segment of 100 mm, with a score of 0 on the left representing no pain, and a score of 10 on the right representing unbearable pain, and the higher the score, the more severe the pain. Patients subjectively judge the degree of knee pain to determine the pain score.
Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score
Adopt internationally recognized WOMAC score system, which is an internationally recognized evaluation scale of osteoarthritis, which mainly includes pain (20 points), stiffness (8 points) and daily functional activities (68 points). The score is 0–96 points. The higher the total score is, the more serious the knee joint dysfunction is. The five-grid scoring method of V3.1 Putonghua version will be used in the study.
Knee Outcome Survey Activities of Daily Living Scale (KOS-ADLS) score
The simplified Chinese version of KOS-ADLS with good reliability and validity will be used for detection. KOS-ADLS is a self-reported test for knee joint dysfunction and dysfunction in patients with daily life activities. 7 items of KOS-ADLS are related to other symptoms and 10 items are related to functional disability in activities of daily life. Each item has a score of 0-5, and the total score is expressed as a percentage. The lower the score, the more serious the degree of disability (11).
Secondary end points
Time up and go test (TUG)
Sitting on a chair with armrests, the patient will be asked to stand up, reach the location of three meters as quickly and safely as possible, and then return to sit down again, the whole process will be timed in seconds. Less than 10 seconds indicates normal activity, 11 to 20 seconds is the normal range for the frail elderly and disabled, and more than 20 seconds indicates the need for help, and the need for further examination and intervention.
6-minute walking test
Patients will be asked to move forward as much as possible within a 6-minute time range and can stop and rest if necessary. Standardized verbal encouragement will be given at 60-second intervals: “you’re doing well. Keep it that way.” Patients can use normal walking aids if necessary. The test will be carried out on a barrier-free horizontal road, and the distance the patient walk will be measured in meters.
Stair test
Patients will be asked to go up and down 9 stairs in their daily way, each step is 20cm, the pace is comfortable and safe, and the whole process will be timed in seconds.
Range of motion (ROM)
(I) Passive range of motion of the knee joint: use a protractor, with one arm parallel to the long axis of the femur from the great trochanter, and the other arm parallel to the long axis from the lateral malleolus of the fibula, and its axis point placed on the lateral condyle of the femur. Knee straightening: the patient lies on his back, the heel of the measuring side is placed on the pad, so that there is no support behind the knee joint and calf, and the degree of knee straightening is recorded with a goniometer; knee flexion: the patient lies on his back and flexion the knee actively, and then the therapist assists him with passive knee flexion to the position of tissue resistance, and the degree of flexion of the knee joint is recorded with a goniometer; and (II) active range of motion of the knee joint: use a protractor, with one arm parallel to the long axis of the femur from the great trochanter, and the other arm parallel to the long axis from the lateral malleolus of the fibula, and its axis point placed on the lateral condyle of the femur. Knee straightening: the patient lies on his back, and the heel of the measuring side is placed on the pad, so that there is no support behind the knee joint and calf. Tell the patient to contract the quadriceps femoris and record the degree of knee joint straightening with a protractor; knee flexion: the patient lies on his back and tries his best to flexion the knee actively. Use a protractor to record the degree of knee flexion.
Knee joint isokinetic muscle strength test
The isokinetic muscle strength testing and training system of Fujian University of traditional Chinese Medicine affiliated Rehabilitation Hospital (USA, BTE.Primus.RS) will be used to measure the maximum isokinetic flexion and extension centripetal torque of knee extensor (mainly quadriceps femoris) and flexor (mainly hamstring). The sample size will be 50 cases. The angular velocity will be 60°/s and the flexion angle of knee joint will be 20° to 90°. The subjects will first perform 5 maximal isokinetic contraction tests. After resting for 2 min, the subjects will be asked to perform 3 groups of 5 maximal isokinetic flexion and extension centripetal contractile tests, and the highest values will be recorded. The testing process will be completed by the same researchers with a unified password. The evaluation indexes will be peak work, absolute peak torque of flexor and extensor (aPT, Nm), relative peak torque of flexion and extensor (rPT, Nm/kg), relative peak moment of flexion/extensor (flexor/extensor), that is, the ratio of relative peak torque of flexor to extensor.
Safety end points
Safety outcomes
Safety outcomes mainly include routine blood and urine test; liver and kidney function; lateral radiographs of both knees and so on.
Incidence rate of side effects
The side effects of different treatment schemes will be recorded and their correlation with the corresponding treatment schemes will be evaluated.
Safety evaluation
The incidence rate of side effects of different treatment schemes will be statistically compared, and the safety of different treatment schemes will be evaluated.
Data management and statistical analysis
Filling and keeping of research medical records and study case observation form (CRF form)
- All selected cases will be asked to complete the research medical records and CRF form in strict accordance with the requirements, and the operators and filling personnel should sign and confirm on the spot for the data filling and evaluation part. Source documents: detailed study medical records include: informed consent, subjects’ treatment process records, curative effect evaluation records, original data of various indicators (photocopies of the report form), serious adverse events report forms, memos.
- Source data: the CRF table of the source data of this subject includes: subject name, age, sex, height, weight, heart rate, blood pressure, address, contact number, name of the research center, enrollment and program number, trial start date, treatment plan, evaluation date and results, signature of evaluators and researchers, serious adverse events and their handling, signature of monitors.
- All the patients who have been selected to meet the inclusion criteria and fill in the informed consent form should record all the items in the research medical records and CRF form in a careful, detailed and timely manner, and submit and save them in time after filling them out; those items that need to be changed at will and without reason should record the reason and date of the change, and be signed by the corrector. For the cases that are not completed in accordance with the requirements in the course of the study, the specific reasons and time should be recorded in detail for the final statistical analysis of the data.
Medical records and source data management
- As the first-hand data in the records of clinical research data, the basic data, evaluation results and other observed results of patients should be recorded timely, accurately, completely, standardized and truly. The research medical records will be completed by the main researchers and research members, and kept by the professional departments of the research institutions.
- The preservation of research medical records stipulates that the research medical records shall be kept in clinical research institutions by special persons. After the completion of the project, all research cases should be transferred to the Affiliated Rehabilitation Hospital of Fujian University of traditional Chinese Medicine for centralized filing.
- Security of research cases: define access to research cases and source data. Preservation period of study cases: according to the regulations of GCP and medical records, the preservation period of study cases in this project is 5 years after the completion of the research work.
- Access to research cases: follow the regulations on access to medical documents and documents in the archives of clinical research institutions.
Data analysis
- Shedding cases: for the cases that lost follow-up in the middle of the study, the shedding medical records will be not included in the statistics, but the specific causes and dates of shedding should be recorded.
- Exclusion of medical records: for the cases that did not strictly follow the requirements of the research program in the course of the study, although the whole research process will be completed, full consideration will be given to the cases in which the statistical analysis of the results would deviate or even seriously interfere with the analysis of the results. to be excluded, not included in the final statistical analysis of the main curative effect observation.
- Full Analysis Set (FAS): refers to the collection of qualified cases and shedding cases, but does not include excluded cases. The basic data are compared and analyzed according to the actual data obtained in FAS.
- Per Protocol Set (PPS): patients who are in accordance with the experimental scheme, good compliance and completed the contents of CRF will be analyzed (PP analysis), which is mainly used for the main indicators of curative effect.
- Safety Set (SS): patients receiving at least one treatment and having actual data recorded by safety indicators. Missing security values must not be carried forward.
Statistical analysis content
- Comparability analysis and compliance analysis of basic data: comparability analysis compares demographic data and other basic value indicators to compare the balance between the two groups; compliance analysis compares whether it is carried out according to the study scheme and whether to use the treatment prohibited in the scheme.
- Therapeutic index analysis: analysis of the factors affecting the curative effect, such as age, sex, family support and so on.
- Safety index analysis: firstly, according to the requirements of the correlation of adverse reactions, the adverse events and adverse reactions of each group will be described, and the reasons will be explained. Chi-square test will be used to analyze the incidence of adverse events in each group.
Statistical method
- Statistical analysis: all data are entered into Excel database by 2 authors independently, with divergences checked and verified. The entry personnel will not participate in case distribution, clinical treatment and result evaluation. After the data entry is completed, a third-party research institution is entrusted to carry out data statistical analysis and random number management to ensure the blind method of statistical analysis. SPSS20.0 software will be used for statistics, and the related outcome indexes and curative effect indexes will be tested by bilateral test, the statistical results will be considered to be statistically significant, with P<0.01 indicating significant statistical significance.
- One-Sample Kolmogorov-Smirnov Test will be used to test the normality of data. The measurement data that accord with the normal distribution are expressed by the mean ± standard deviation, and will be further entered to the variance homogeneity Levene test (Levene’s test for homogeneity of variance); data not conformed to the normal distribution is expressed by the median (M) and 25–75% quartile (Quartiles). Paired measurement data with normal distribution and uniform variance will be compared by paired t-test, and paired symbolic rank sum test will be used to compare paired measurement data that did not conform to normal distribution. The measurement data of completely random design in accordance with normal distribution will be compared by group t-test (the results of t-test for variance and correction t-test for variance), and the measurement data of completely random design that did not accord with normal distribution will be compared with Mann-Whitney U test or Wilcoxon rank sum test. The counting data will be compared by χ2 test and described statistically. Multiple groups of samples will be tested for analysis of variance and homogeneity of variance, and pairwise comparison will be carried out. When the variance will be uniform, look at the LSD table, and when the variance will be uneven, look at the Games-Howell table.
- Confounding factor analysis: the observation index of curative effect and the index of outcome, such as the imbalance between groups, will be analyzed by unbalanced factors.
Ethical statement
All procedures performed in this study involving human participants will in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics committee of Second Department of Orthopedic Rehabilitation, Rehabilitation Hospital Affiliated to Fujian University of Traditional Chinese Medicine and informed consent will be taken from all the patients.
Discussion
The occurrence mechanism of knee osteoarthritis is still unclear, and we hypothesized that traditional Chinese medicine can regulate the body, enhance immunity and improve the blood supply of cartilage, so as to effectively treat knee osteoarthritis. We believe the proposed randomized controlled study will provide important data for the future treatment guidelines for KOA. The above steps will be strictly implemented to ensure the reliability of our results. The study data may provide KOA patients with more options to relieve their symptoms.
Acknowledgments
Funding: Key Laboratory of Orthopedics & Traumatology of Traditional Chinese Medicine and Rehabilitation Ministry of Education, Fujian University of TCM (No. ZD2020-2-2); National Administration of Traditional Chinese Medicine: 2019 Project of building evidence based practice capacity for TCM (No. 2019XZZY-GK001); Fujian Key Laboratory of Rehabilitation Technology (No. KF2019027).
Footnote
Reporting Checklist: The authors have completed the SPIRIT reporting checklist. Available at https://dx.doi.org/10.21037/apm-21-1179
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apm-21-1179). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants will in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics committee of Second Department of Orthopedic Rehabilitation, Rehabilitation Hospital Affiliated to Fujian University of Traditional Chinese Medicine and informed consent will be taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zeng SY, Gong Y, Zhang YP, et al. Changes in the Prevalence of Rheumatic Diseases in Shantou, China, in the Past Three Decades: A COPCORD Study. PLoS One 2015;10:e0138492 [Crossref] [PubMed]
- Barbour KE, Stevens JA, Helmick CG, et al. Falls and fall injuries among adults with arthritis--United States, 2012. MMWR Morb Mortal Wkly Rep 2014;63:379-83. [PubMed]
- Sharma L. Osteoarthritis year in review 2015: clinical. Osteoarthritis Cartilage 2016;24:36-48. [Crossref] [PubMed]
- Li XH, Liang WN, Liu XX. Clinical observation on curative effect of dissolving phlegm-stasis on 50 cases of knee osteoarthritis. J Tradit Chin Med 2010;30:108-12. [Crossref] [PubMed]
- Lee HJ, Park HJ, Chae Y, et al. Tai Chi Qigong for the quality of life of patients with knee osteoarthritis: a pilot, randomized, waiting list controlled trial. Clin Rehabil 2009;23:504-11. [Crossref] [PubMed]
- Brismée JM, Paige RL, Chyu MC, et al. Group and home-based tai chi in elderly subjects with knee osteoarthritis: a randomized controlled trial. Clin Rehabil 2007;21:99-111. [Crossref] [PubMed]
- Williamson L, Wyatt MR, Yein K, et al. Severe knee osteoarthritis: a randomized controlled trial of acupuncture, physiotherapy (supervised exercise) and standard management for patients awaiting knee replacement. Rheumatology (Oxford) 2007;46:1445-9. [Crossref] [PubMed]
- Hou PW, Fu PK, Hsu HC, et al. Traditional Chinese medicine in patients with osteoarthritis of the knee. J Tradit Complement Med 2015;5:182-96. [Crossref] [PubMed]
- Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200-7. [Crossref] [PubMed]
- Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage 2019;27:1578-89. [Crossref] [PubMed]
- Jia ZY, Wang W, Nian XW, et al. Cross-cultural Adaptation and Validation of the Simplified Chinese Version of the Knee Outcome Survey Activities of Daily Living Scale. Arthroscopy 2016;32:2009-16. [Crossref] [PubMed]
(English Language Editor: R. Scott)


