
Successful treatment using targeted therapy, radiotherapy, and intrathecal chemotherapy in a patient with leptomeningeal metastasis with an epidermal growth factor receptor exon 20 insertion mutation: a case report
Introduction
Since the epidermal growth factor receptor (EGFR) exon20ins mutation was first described in non-small cell lung cancer (NSCLC) in 2004 (1,2), over 100 potential mutations have been identified (3). This highly heterogeneous structure displays diverse biological behaviors and different responses to targeted therapy (4). With the exception of A763_Y764insFQEA (1,5), the EGFR exon20ins mutation was viewed as de novo resistant to tyrosine kinase inhibitors (TKIs) (4), and conventional chemotherapy (CT) has been the mainstay of its management. Additionally, leptomeningeal metastasis (LM) remains challenging to treat, despite modern strategies that have improved patients’ overall survival (OS) time from 1–3 to 3–11 months (6,7). Brastianos et al. revealed that in 53% of patients with brain metastasis (BM), a specific genetic alternation developed in the BM was not detected in the matched primary tumor (8). Cerebrospinal fluid (CSF) next-generation sequencing (NGS) also documented different genetic landscapes, such as less T790M and mesenchymal‐to‐epithelial transition (MET) activation by MET copy number gain (9,10). Thus, CSF NGS provides valuable information for treating these 2 difficult problems clinically. The patient in this case presented with aggressive LM with an EGFR exon20ins mutation. The following case is presented following the CARE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-21-321/rc).
Case presentation
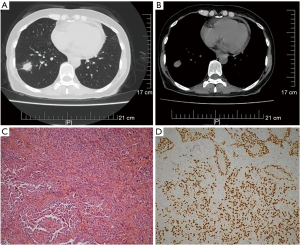
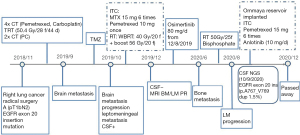
A 56-year-old female non-smoker presented in November 2018 after a lump located in the lower lobe of the right lung (see Figure 1A,1B) and enlarged mediastinal lymph nodes were found in a routine physical examination. The initial evaluations of the patient’s abdomen, bone, and brain were unremarkable. The patient’s clinical-stage was IIIA cT1bN2M0. Radical surgery was performed on the patient. The patient’s postoperative pathology showed an invasive adenocarcinoma of 1.8×1.8×1.5 cm (solid type: 70%; papillary type: 30%) (see Figure 1C,1D) with multiple mediastinal lymph node involvement (stations: 2: 2/2; 3: 6/8; 3p: 1/1; 4: 2/2; 7: 3/7). The patient’s final pathological stage was III A (T1bN2). The EGFR mutation assessment revealed an EGFR exon 20 insertion mutation without an anaplastic lymphoma kinase (ALK) rearrangement and ROS1 fusion. According to the International Adjuvant Lung Cancer Trial (IALT), cisplatin-based chemotherapy brought both 5-y OS (45% vs. 40%, P<0.03) and 5-y DFS (39% vs. 34%, P<0.003) benefit comparing with observation in completely resected stage I-III NSCLC (11). Lung Adjuvant Cisplatin Evaluation (LACE) Collaborative Group (a meta-analysis of 4584 patients) also verified a 5-year OS improvement (a 5.4% absolute benefit) following postoperative cisplatin-based CT (12). Further, the TREAT study showed that cisplatin/pemetrexed was a less toxic regimen than cisplatin/vinorelbine and had similar efficacy in completely resected stage IB–III NSCLC patients. Thus, patients could endure more cycles of CT with cisplatin/pemetrexed (13,14). A series of studies have demonstrated that postoperative radiotherapy (RT) improves the survival of stage III–pN2 NSCLC patients (15-17), especially among those with a high metastatic mediastinal lymph node ratio (MLNR) ≥50% (18,19). The patient was at pathological stage III A (pT1bN2). Based on the positivity of the highest resected mediastinal lymph node and a MLNR of 70%, the patient was given postoperative CT and RT. Specifically, the patient underwent 4 cycles of adjuvant CT (PC: Pemetrexed, Carboplatin) followed by thoracic irradiation (50.4 Gy/28 f/44 d, 4/15/2019-5/22/2019) and 2 more cycles of PC CT.
On May 24, 2019, brain magnetic resonance imaging (MRI) with contrast was performed, and metastasis was excluded. In September, the patient suffered from mild headaches and dizziness for a week. On September 18, 2019, another brain MRI with contrast was performed, and 3 metastatic lesions about 0.3–0.4 cm located in the bilateral frontal lobes and the right temporal lobe were found (see Figure 2A-2C). Temozolomide (TMZ) was able to cross the blood-brain barrier. A meta-analysis showed that the addition of TMZ to whole-brain radiotherapy (WBRT) resulted in an increased overall response rate (ORR) among NSCLC patients with BM compared to those treated with WBRT alone (11). The tertiary hospital initiated TMZ for 1 cycle (TMZ 200 mg d1–5). However, the patient’s brain MRI with the contrast of October 27, 2019, showed an enlargement of the BM above and the onset of the metastatic meningeal lesion (see Figure 2D-2F).

The CSF evaluation showed normal biochemical results except for a slightly increased leukocyte count of 11×106/L [0–8]; however, malignant cells were found. The NGS of the postoperative tumor tissue revealed an EGFR exon 20 insertion mutation (p. V769_D770 ins ASV: 17.48%), tumor mutational burden (TMB): 3.94 Muts/Mb,microsatellite stable (MSS), TP53 mutation: 4.71%. The PD-L1 expression of the primary tumor had a tumor proportion score (TPS) of 5%.
The patient underwent intrathecal chemotherapy (ITC) with methotrexate (MTX) 6 times (15 mg, 10/31/2019, 11/7/2019, 11/12/2019, 11/19/2019, 11/26/2019, 12/3/2019) and pemetrexed once (10 mg, 12/19/2019), and the CSF tumor cells turned negative (on December 19, 2019). The patient also received WBRT (40 Gy/2 Gy/20 f)and a tumor boost (56 Gy/2.8 Gy/20 f) from October 31, 2019, to November 27, 2019. With the contrast of December 5, 2019, the brain MRI showed a partial response (PR) by the BM and LM lesions (see Figure 2G-2I). Osimertinib 80 mg daily was initiated from December 8, 2019.
On June 12, 2020, the patient was referred to our department as she experienced pain and numbness in both lower limbs for 2 weeks. The subsequent lumbosacral spine MRI scan revealed multiple metastases from lumbar 3 to sacral 2 vertebrae, appendages, and bilateral iliac bones. A bone scan also manifested increased radioactivity on the 9th right rib, lumbar 3–5 vertebrae, sacrum, and left iliac bone, which was considered bone metastasis. The brain MRI with a contrast of June 16, 2020, showed lesions in the bilateral frontal lobes, right temporal lobe, and the meninges of the right frontal lobe had disappeared. Anlotinib (10 mg/d) was administered for less than 14 days and then suspended due to nausea. RT to the lumbosacral and pelvic bone metastases was performed with a dose of 50 Gy/2 Gy/25 f from June 23, 2020, to July 30, 2020. Bisphosphate was administered monthly, and the patient’s pain was greatly relieved. After that, the patient suffered from vulvar infection, which resolved after anti-inflammatory and symptomatic supportive treatment.
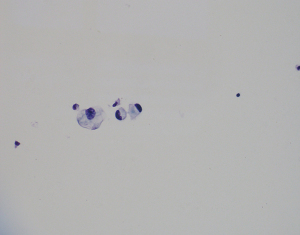
On August 19, 2020, the patient complained of a headache. A cranial CT scan excluded encephalorrhagia. The CSF evaluation of August 21, 2020, showed adenocarcinoma cells again just as on October 28, 2019, the baseline CSF smear demonstrated malignant cells (see Figure 3). The subsequent brain MRI with contrast revealed abnormal thickening and an enhanced cerebellar pial membrane (see Figure 4). An intraventricular Ommaya reservoir was implanted, and pemetrexed was administered 6 times (15 mg on 8/28, 9/11, 9/20, 9/27, 10/5, and 10/20) via the Ommaya reservoir, and resulted in negative CSF cytology. Anlotinib (10 mg/d) was administered on August 23, 2020. The CSF NGS examination of October 9, 2020 showed a different EGFR exon20ins (p. A767_V769 dup: 1.5%) and TP53 mutation (0.3%). The patient succumbed to the disease on December 7, 2020. The patient’s OS time after LM diagnosis was 13.5 months (see Figure 5).
All the procedures in this study involving the human participant were performed following the ethical standards of the institutional and/or national research committee(s) and the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s son to publish this manuscript and the accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
LM is generally considered a fatal and disastrous condition in lung cancer. Patients with EGFR-mutated lung cancer have a higher incidence of LM than their EGFR wild-type counterparts (9.4% vs. 1.7%) (7). How to screen populations at high risk of developing LM is critical to clinical decision-making. The precise determination of the related “high-risk” factors will guarantee close follow-up appointments, timely examinations, and the early detection and correct management of this special entity. Boire et al. revealed that the complement component 3 (C3) pathway played a key role in LM. Cancer cell-derived C3 disrupted the blood-CSF barrier by activating the C3a receptor located in the epithelium of the choroid plexus (12).
The EGFR exon20ins mutation accounts for approximately 3.6–13% of all EGFR mutations (4,13-16). EGFR exon20ins mutations have various subtypes, most of which are resistant to first- and second-generation EGFR TKIs (14) (except the EGFR A763_Y764insFQEA). According to a report by Fang et al., A767_V769dup is the most common EGFR exon20ins type and comprises 32.1% (17/53) of all EGFR exon20ins (17). Additionally, EGFR exon20ins appear to be exclusive with other important driver genes, including ALK, BRAF, RET, and ERBB2 (17). TP53 is a common co-mutation (49.1%) (17). In our patient, the NGS of the primary tumor revealed an EGFR exon20ins mutation (p. V769_D770 ins ASV: 17.48%) with a TP53 mutation (4.71%). The CSF NGS showed different EGFR exon20ins (p. A767_V769 dup: 1.5%) and TP53 mutations (0.3%).
CSF NGS is fundamental in diagnosis, gene landscaping, determining treatment options, and evaluating responses. The circulating tumor cells (CTC) in CSF have a sensitivity of 94%, which is higher than that of cytology, which has a sensitivity of 76% (18). Thus, CSF CTC can be used to identify patients with LM more accurately (18).
Liu et al. described a stage IV NSCLC patient with BM with EGFR exon21 L858R, who developed LM after the first-line treatment of icotinib. Osimertinib was then initiated for acquired T790M. However, after 9 months of Osimertinib treatment, the LM had progressed. Further, the CSF NGS revealed an L718Q mutation. The switch to afatinib correspondingly controlled the disease for 4 months (19). In our patient, the CNS NGS revealed a different EGFR exon20ins variant (p. A767_V769 dup: 1.5%) from that of the primary tumor (p. V769_D770 ins ASV: 17.48%). This emphasizes the importance of liquid biopsies of CSF NGS for the precise management of LM, as tumor cells mutate genetically to adapt to new microenvironments. The management of the EGFRexon20ins mutation is challenging, and to date, no standard has been established. van Veggel et al. found that the intensification of the EGFR blockade by afatinib and cetuximab in 4 patients harboring the EGFRexon20ins mutation achieved a PR in 3 patients and a stable disease (SD) response in 1 patient, and a median progression-free survival (PFS) time of 5.4 months. However, the common side effects, such as skin toxicity and diarrhea, caused by both afatinib and cetuximab add up, and a dose reduction was required in 2 patients (20).
The third-generation EGFR TKI-Osimertinib has shown certain efficacy concerning the EGFRexon20ins mutation in preclinical models (21). According to a retrospective study of 6 patients with EGFR exon20ins mutations, the administration of 80 mg of Osimertinib daily achieved a PR in 4 patients (p. A767_V769dup: 3.48%; p. S768_D770dup: 1.75%; p. D770_N771insG: 24%; p. A763_Y764 ins FQEA: 0.2% with T790 M: 0.3%) and a SD response in 2 patients (p. N771_P772insL: 47%; p. S768_D770dup: 3%) with a median PFS time of 6.2 months (17). Floc'h et al. established CRISPR-Cas 9 engineered cell lines with common EGFR exon20ins mutations (D770_N771InsSVD or V769_D770InsASV) and verified the efficacy of Osimertinib in vitro and in vivo xenograft models (3). Poziotinib, a potent inhibitor of the EGFR and HER2 exon 20 mutation, had an ORR of 64% (22). The Hsp90 inhibitor Luminespib can degrade EGFR exon 20 mutations (23) and showed a 17% ORR in a phase II trial of NSCLC patients carrying the EGFR ins20 (24). TAS6417/CLN-081 had better efficacy for EGFR exon20ins mutations than Poziotinib (25). Based on the availability of agents, our patient was treated with Osimertinib combined with ITC, RT, and anti-angiogenesis drugs and achieved a 13.5-month OS.
In the management of LM, local RT aims to control bulky or nodular lesions, correct CSF flow, helping ITC to exert the effect (9). Presently, there is no consensus about the role of WBRT in LM (9), especially in the oncogene-addicted population with CNS-potent target agents. In their report, Li et al. found that the addition of WBRT failed to add any survival benefits to LM patients harboring EGFR common mutations who received TKI alone (7). Yan et al. found no difference in intracranial ORR and DCR between a WBRT and non-WBRT group in 51 EGFR-mutated LM NSCLC patients (19del: 20; 21 L858R: 31) (26). Morris et al. reviewed 125 NSCLC patients who developed LM (9: known EGFR mutation) but found no survival difference between WBRT (n=46) and non-WBRT patients (n=59; P=0.84) (27). Conversely, Liao et al. revealed that WBRT was an independent factor for a better prognosis among LM patients (28). Wu et al. performed a single institutional analysis of 420 patients who had received first-generation EGFR TKI over 6 months and found that 29 of the 420 patients suffered from LM after a median duration of 16.5 months. Multivariate analysis indicated that WBRT was associated with improved OS (P=0.048) (29). Given the rapid progression of LM and the unavailability of other target agents, including Poziotinib, TAK788, WBRT (40 Gy/20 f) with a simultaneous integrated boost (SIB) (56 Gy/20 f) followed by Osimertinib was initiated. Radical local treatment (WBRT with a SIB) controlled the LM to a certain extent. Concurrent ITC with 15 mg of MTX (6 times) and 10 mg Pemetrexed (once) led to CSF cytological negativity.
The intraventricular Ommaya reservoir provides better CT agent distribution and is safer and more convenient than a conventional lumbar puncture (30). ITC with pemetrexed exerted a certain efficacy in NSCLC patients with LM, especially those resistant to TKIs (31). Miao et al. reported that intrathecal pemetrexed–based multimodal treatment provided reasonable control of refractory LM with tolerable toxicity (32). Anlotinib, as a novel multitarget TKI for tumor proliferative signal and anti-angiogenesis (VEGFR, PDGFR, FGFR, c-Kit) (33), might help to reverse treatment resistance partially. In a further analysis of ALTER0303 (34), anlotinib was shown to prolong the time to brain progression significantly. In the refractory LM setting, 15 mg of pemetrexed (6 times) was delivered by the intraventricular Ommaya reservoir with 10 mg of Anlotinib and 80 mg of Osimertinib (daily) turned the patient’s CSF cytology negative again and further extended the patient’s OS by 4 months.
The role of the immune checkpoint inhibitor (ICI) in LM has not yet been established. Only a few cases have reported on patients’ responses to ICI in the LM setting (35). The NGS of the primary tumor showed MSS status, low TMB, and PD-L1 expression concerning our patient. Anti-inflammatory treatment was given to control the patient’s vulvar infection. It is unlikely that the patient would have benefited from ICI.
Conclusions
In this article, we presented the case of a patient with refractory LM NSCLC harboring the EGFR exon20ins mutation. NGS of both the primary tumor and CSF enabled a systemic therapy to be tailored for the patient, and the multimodalities conferred a post-LM OS of 13.5 months. The findings, in this case, are noteworthy for clinicians. Further attention needs to be paid to populations at high risk of developing LM. It is strongly recommended that patients suffering from BM, especially those with indicative radiologic signs of LM, such as linear or nodular leptomeningeal enhancement, BM near the leptomeninges, brain ventricle or in the sulcus, undergo a liquid biopsy of CSF for early diagnosis, genetic profile identification, and a corresponding systemic therapy for LM. Additionally, multidisciplinary modality is vital for a better prognosis. MTX/pemetrexed delivered by the Ommaya reservoir, local RT, CSF NGS guided target therapy, and even ICI can prolong patients’ survival significantly.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-21-321/rc
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-21-321/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-21-321/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work, including ensuring that all questions related to the accuracy or integrity of any part of the work have been appropriately investigated and resolved. All the procedures in this study involving the human participant were performed following the ethical standards of the institutional and/or national research committee(s) and the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s son to publish this manuscript and the accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vyse S, Huang PH. Targeting EGFR exon 20 insertion mutations in non-small cell lung cancer. Signal Transduct Target Ther 2019;4:5. [Crossref] [PubMed]
- Kosaka T, Yatabe Y, Endoh H, et al. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res 2004;64:8919-23. [Crossref] [PubMed]
- Floc'h N, Martin MJ, Riess JW, et al. Antitumor Activity of Osimertinib, an Irreversible Mutant-Selective EGFR Tyrosine Kinase Inhibitor, in NSCLC Harboring EGFR Exon 20 Insertions. Mol Cancer Ther 2018;17:885-96. [Crossref] [PubMed]
- Arcila ME, Nafa K, Chaft JE, et al. EGFR exon 20 insertion mutations in lung adenocarcinomas: prevalence, molecular heterogeneity, and clinicopathologic characteristics. Mol Cancer Ther 2013;12:220-9. [Crossref] [PubMed]
- Voon PJ, Tsui DW, Rosenfeld N, et al. EGFR exon 20 insertion A763-Y764insFQEA and response to erlotinib--Letter. Mol Cancer Ther 2013;12:2614-5. [Crossref] [PubMed]
- Yin K, Li YS, Zheng MM, et al. A molecular graded prognostic assessment (molGPA) model specific for estimating survival in lung cancer patients with leptomeningeal metastases. Lung Cancer 2019;131:134-8. [Crossref] [PubMed]
- Li YS, Jiang BY, Yang JJ, et al. Leptomeningeal Metastases in Patients with NSCLC with EGFR Mutations. J Thorac Oncol 2016;11:1962-9. [Crossref] [PubMed]
- Brastianos PK, Carter SL, Santagata S, et al. Genomic Characterization of Brain Metastases Reveals Branched Evolution and Potential Therapeutic Targets. Cancer Discov 2015;5:1164-77. [Crossref] [PubMed]
- Cheng H, Perez-Soler R. Leptomeningeal metastases in non-small-cell lung cancer. Lancet Oncol 2018;19:e43-e55. [Crossref] [PubMed]
- Nanjo S, Arai S, Wang W, et al. MET Copy Number Gain Is Associated with Gefitinib Resistance in Leptomeningeal Carcinomatosis of EGFR-mutant Lung Cancer. Mol Cancer Ther 2017;16:506-15. [Crossref] [PubMed]
- Xin Y, Guo W, Yang CS, et al. Meta-analysis of whole-brain radiotherapy plus temozolomide compared with whole-brain radiotherapy for the treatment of brain metastases from non-small-cell lung cancer. Cancer Med 2018;7:981-90. [Crossref] [PubMed]
- Boire A, Zou Y, Shieh J, et al. Complement Component 3 Adapts the Cerebrospinal Fluid for Leptomeningeal Metastasis. Cell 2017;168:1101-13.e13. [Crossref] [PubMed]
- Tu HY, Ke EE, Yang JJ, et al. A comprehensive review of uncommon EGFR mutations in patients with non-small cell lung cancer. Lung Cancer 2017;114:96-102. [Crossref] [PubMed]
- Yasuda H, Kobayashi S, Costa DB. EGFR exon 20 insertion mutations in non-small-cell lung cancer: preclinical data and clinical implications. Lancet Oncol 2012;13:e23-31. [Crossref] [PubMed]
- Naidoo J, Sima CS, Rodriguez K, et al. Epidermal growth factor receptor exon 20 insertions in advanced lung adenocarcinomas: Clinical outcomes and response to erlotinib. Cancer 2015;121:3212-20. [Crossref] [PubMed]
- Sasaki H, Endo K, Takada M, et al. EGFR exon 20 insertion mutation in Japanese lung cancer. Lung Cancer 2007;58:324-8. [Crossref] [PubMed]
- Fang W, Huang Y, Hong S, et al. EGFR exon 20 insertion mutations and response to osimertinib in non-small-cell lung cancer. BMC Cancer 2019;19:595. [Crossref] [PubMed]
- van Bussel MTJ, Pluim D, Milojkovic Kerklaan B, et al. Circulating epithelial tumor cell analysis in CSF in patients with leptomeningeal metastases. Neurology 2020;94:e521-8. [Crossref] [PubMed]
- Liu J, Jin B, Su H, et al. Afatinib helped overcome subsequent resistance to osimertinib in a patient with NSCLC having leptomeningeal metastasis baring acquired EGFR L718Q mutation: a case report. BMC Cancer 2019;19:702. [Crossref] [PubMed]
- van Veggel B, de Langen AJ, Hashemi SMS, et al. Afatinib and Cetuximab in Four Patients With EGFR Exon 20 Insertion-Positive Advanced NSCLC. J Thorac Oncol 2018;13:1222-6. [Crossref] [PubMed]
- Hirano T, Yasuda H, Tani T, et al. In vitro modeling to determine mutation specificity of EGFR tyrosine kinase inhibitors against clinically relevant EGFR mutants in non-small-cell lung cancer. Oncotarget 2015;6:38789-803. [Crossref] [PubMed]
- Robichaux JP, Elamin YY, Tan Z, et al. Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer. Nat Med 2018;24:638-46. [Crossref] [PubMed]
- Jorge SE, Lucena-Araujo AR, Yasuda H, et al. EGFR Exon 20 Insertion Mutations Display Sensitivity to Hsp90 Inhibition in Preclinical Models and Lung Adenocarcinomas. Clin Cancer Res 2018;24:6548-55. [Crossref] [PubMed]
- Piotrowska Z, Costa DB, Oxnard GR, et al. Activity of the Hsp90 inhibitor luminespib among non-small-cell lung cancers harboring EGFR exon 20 insertions. Ann Oncol 2018;29:2092-7. [Crossref] [PubMed]
- Udagawa H, Hasako S, Ohashi A, et al. TAS6417/CLN-081 Is a Pan-Mutation-Selective EGFR Tyrosine Kinase Inhibitor with a Broad Spectrum of Preclinical Activity against Clinically Relevant EGFR Mutations. Mol Cancer Res 2019;17:2233-43. [Crossref] [PubMed]
- Yan W, Liu Y, Li J, et al. Whole brain radiation therapy does not improve the overall survival of EGFR-mutant NSCLC patients with leptomeningeal metastasis. Radiat Oncol 2019;14:168. [Crossref] [PubMed]
- Morris PG, Reiner AS, Szenberg OR, et al. Leptomeningeal metastasis from non-small cell lung cancer: survival and the impact of whole brain radiotherapy. J Thorac Oncol 2012;7:382-5. [Crossref] [PubMed]
- Liao BC, Lee JH, Lin CC, et al. Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors for Non-Small-Cell Lung Cancer Patients with Leptomeningeal Carcinomatosis. J Thorac Oncol 2015;10:1754-61. [Crossref] [PubMed]
- Wu YL, Zhao Q, Deng L, et al. Leptomeningeal metastasis after effective first-generation EGFR TKI treatment of advanced non-small cell lung cancer. Lung Cancer 2019;127:1-5. [Crossref] [PubMed]
- Lin Y, Li H, Huang M, et al. Zhongguo Fei Ai Za Zhi 2019;22:546-50. [Use of Ommaya Reservoirs to Deliver Pemetrexed in Leptomeningeal Metastasis from Non-small Cell Lung Cancer: A Case Report and Review of the Literature]. [PubMed]
- Li H, Lin Y, Yu T, et al. Treatment response to intrathecal chemotherapy with pemetrexed via an Ommaya reservoir in EGFR-mutated leptomeningeal metastases from non-small cell lung cancer: a case report. Ann Palliat Med 2020;9:2341-6. [Crossref] [PubMed]
- Miao Q, Zheng X, Zhang L, et al. Multiple combination therapy based on intrathecal pemetrexed in non-small cell lung cancer patients with refractory leptomeningeal metastasis. Ann Palliat Med 2020;9:4233-45. [Crossref] [PubMed]
- Han B, Li K, Wang Q, et al. Effect of Anlotinib as a Third-Line or Further Treatment on Overall Survival of Patients With Advanced Non-Small Cell Lung Cancer: The ALTER 0303 Phase 3 Randomized Clinical Trial. JAMA Oncol 2018;4:1569-75. [Crossref] [PubMed]
- Jiang S, Liang H, Liu Z, et al. The Impact of Anlotinib on Brain Metastases of Non-Small Cell Lung Cancer: Post Hoc Analysis of a Phase III Randomized Control Trial (ALTER0303). Oncologist 2020;25:e870-4. [Crossref] [PubMed]
- Gion M, Remon J, Caramella C, et al. Symptomatic leptomeningeal metastasis improvement with nivolumab in advanced non-small cell lung cancer patient. Lung Cancer 2017;108:72-4. [Crossref] [PubMed]
(English Language Editors: L. Huleatt and J. Chapnick)


