
Cranioplasty for patients with disorders of consciousness
Introduction
Coma may be secondary to severe traumatic brain injury (TBI), cerebral hemorrhage, massive cerebral infarction, or other severe brain injuries. Decompressive craniectomy (DC) is often performed in the early stages of these diseases. Skull defects may present obstacles to rehabilitation and nursing when the disorders of consciousness (DOC) stage is reached, and cranioplasty (CP) is required. CP is generally considered to improve brain function in patients with skull defects (1,2); the approach is relatively safe and can be performed early (3,4). The overall complication rate is 4.8–61.2%, comprising infection, hydrocephalus, seizure, cerebral hemorrhage, epidural collection, and bone flap resorption, and the mortality rate is 0–3.2% (5).
The prognosis of patients with DOC undergoing CP is uncertain because of severe brain damage and complications, some of which remain hidden due to the absence or scarcity of effective indicators (6). The relatively high incidence of hydrocephalus also complicates treatment (7), and has been considered as a possible significant complication among the neurosurgical sequelae for the prognosis of patients with DOC in an international survey (8). Therefore, surgical decisions tend to be conservative, and are rarely supported by the literature.
In the present study, we reviewed cases of titanium CP in patients with DOC in our center, and summarized the clinical outcomes, surgical complications, perioperative observations, and in particular, the treatment of transcalvarial herniation (TCH) and hydrocephalus. We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/apm-21-1822).
Methods
Patient population
This study was approved by the Ethics Committee of The Seventh Medical Center, Chinese PLA General Hospital (approval number 2019-061), and informed consent was obtained from all patients’ surrogates. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Patients with vegetative state/unresponsive wakefulness syndrome (VS/UWS) (9) or minimally conscious state (MCS), according to the Coma Recovery Scale-Revised (CRS-R) (10), who underwent CP in our center between December 2016 and April 2019 were reviewed. The patients were divided into two groups based on the cause of DOC: a TBI group and a non-TBI group. The age, sex, and time of CP (interval between DC and CP <90 days was considered early CP, and ≥90 days was considered late CP) were recorded. The side of the bone window (small bone window was defined as <100 cm2, and large bone window was >100 cm2), level of consciousness (VS/UWS or MCS), and intracranial pressure at the time of admission were also measured.
Surgical management and follow up
All operations were performed under general anesthesia. The original incision was used, and a preformed titanium mesh was fixed under the scalp and temporalis. Subcutaneous drainage was performed for 24–48 hours postoperatively. Postoperative epidural collection was defined as epidural effusion greater than 15 mL, and cerebral hemorrhage was defined as computed tomography (CT)-confirmed cerebral lobar, subarachnoid, or intraventricular hemorrhage. Impaired wound healing was defined as poor wound healing requiring surgical treatment. Death within 1 month after surgery was defined as surgery-related death. All patients were followed-up via telephone or re-examination by an experienced neurologist for 1 year after CP using the Extended Glasgow Outcome Scale (GOSE) (11).
Management of TCH and hydrocephalus
In patients with preoperative TCH [a typical external cerebral herniation after DC (12)], cerebrospinal fluid (CSF) was released via lumbar puncture or lumbar cistern drainage to relieve brain bulging, followed by head bandaging. Irreversible TCH was defined as TCH that relapsed within 48 hours after a full CSF release, and in these cases, the patient should undergo CP with an interoperative lumbar drainage tube. Hydrocephalus was diagnosed and a ventriculoperitoneal shunt (VPS) was placed in patients who met the CT scan diagnostic criteria (13) and had persistent intracranial hypertension or a positive CSF release test result. Patients with or without any CSF drainage device then underwent CP.
A 24-hour clipping test was performed postoperatively to rule out an increase in intracranial pressure or hydrocephalus symptoms in patients with irreversible TCH and intraoperative indwelling of the lumbar cistern drainage tube. The drainage tube was then removed. A CT scan, series of lumbar punctures, and one or more CSF release tests were performed within 2 weeks after the surgery to assess for the presence of hydrocephalus. A VPS was placed in all patients diagnosed with postoperative hydrocephalus.
Statistical analysis
Analyses were performed using SPSS 24.0 (IBM Company, New York, NY, USA). For intergroup comparisons, the categorical data were analyzed using the chi-square test or Fisher’s exact test. Quantitative data were presented as means ± standard deviations. The preoperative and postoperative intracranial pressures were compared using the paired t-test, and other data were compared using the unpaired t-test. Binary logistic regression was used for the multivariate analysis. All tests were two-sided, and the results were considered statistically significant at P<0.05.
Results
Demographic and clinical characteristics
In this study, 87 patients (67 males and 20 females) with DOC who underwent CP were included. The mean age of included patients was 47.1±14.0 years. Among the 87 cases, 56 were associated with TBI, 30 with intracerebral hemorrhage, and one with massive cerebral infarction. Sixty-nine patients were in a VS/UWS, and 18 were in a MCS. There were 40 left, 33 right, and 14 bilateral skull defects. Skull defects were <100 cm2 in 41 cases and >100 cm2 in 46 cases. Twelve patients (13.8%) underwent VPS due to hydrocephalus; of these, three were performed before admission at the study hospital. The mean interval between DC and CP was 135.8±82.1 (range, 70–566) days, with 63 cases (72.4%) having an interval >90 days.
Postoperative complications occurred in 18 patients, with an incidence of 20.7%. Two patients died within 1-month post-surgery (one had an intracerebral hemorrhage and the other had a severe incision infection). The mortality rate was 2.3%. The average direct cost for CP per patient was 10,912.4±1,789.6 CNY, and the GOSE score averaged 2.4±0.7 points at 1-year postoperatively. The general data were similar between the TBI and the non-TBI groups, but the TBI group had more right and bilateral side CP (P=0.019, P=0.002), a higher overall complication rate (P=0.31), and a higher cost incurred (P=0.003). However, there was no significant difference in the average GOSE score between the two groups (Table 1).
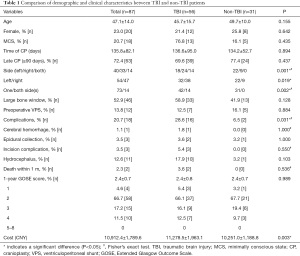
Full table
Clinical outcome
One year after the operation, four patients had died, 58 patients (66.7%) still had DOC (GOSE =2), 15 patients (17.2%) had a GOSE =3, and 10 patients (11.5%) had a GOSE =4 (Table 1). Of the 25 patients who regained full consciousness (GOSE ≥3), 16 were TBI patients, 12 were at a MCS preoperatively, and 10 underwent early CP. Multivariate analysis showed that preoperative MCS was a favorable factor for achieving GOSE ≥3 at the 1-year follow-up, while late CP was a negative factor (P=0.024, Table 2).

Full table
Surgical complications
Among the patients with postoperative complications, 3 (3.5%) presented with epidural collection requiring drainage, 1 (1.1%) with intracerebral hemorrhage, and 3 (3.5%) with impaired wound healing (Table 1). Preoperative VPS was performed in two of the three patients with incision complications and one of the three patients with epidural collection. Multivariate analysis identified preoperative VPS as a risk factor for incision complications (P=0.032, Table 3).

Full table
Management of TCH and postoperative hydrocephalus
A total of 71 patients were included after excluding those with preoperative VPS and postoperative complications (other than hydrocephalus), 11 (15.5%) of whom had postoperative hydrocephalus requiring VPS. Of the 71 patients, 45 (63.4%) had TCH and were treated with CSF release measures (35 patients underwent 1–3 lumbar punctures and 10 patients underwent lumbar drainage), followed by head bandaging. The TCH resolved in 39 patients (maintained for 48 hours after lumbar puncture or drainage clip), with an effectiveness rate of 86.7%. The remaining six patients with irreversible TCH eventually underwent CP with intraoperative lumbar drainage (Figure 1).

All patients underwent lumbar puncture before and after surgery to measure the intracranial pressure. Compared with patients with no hydrocephalus, patients with hydrocephalus had higher intracranial pressure at the first lumbar puncture postoperatively (P=0.000), higher pre- and post-surgery intracranial pressure difference, and a higher proportion of irreversible TCH. However, there was no significant difference in the incidence of reversible TCH between the two groups (Table 4). Multivariate analysis of the 71 patients revealed that non-traumatic causes tended to be protective factors (P=0.052), whereas large bone window and TCH and preoperative CSF pressure were not independent factors (Table 5).

Full table

Full table
Discussion
In our retrospective study, patients were first classified as TBI or non-TBI based on etiology. We found that patients in the TBI group had larger bone windows, more overall complications, and higher costs; however, there was no difference in prognosis between the two groups. Clinical data were further analyzed to understand the clinical outcomes and risk factors for complications, and to evaluate the management of specific conditions, such as TCH.
Clinical outcome
CP for DOC patients is highly indicated for consciousness and cognitive recovery. Since patients are unable to express their wishes, their surrogates’ requests for treatment should be taken into account. Although there is currently no evidence that over-caution in CP affects prognosis, clinicians should avoid making decisions that lead to self-fulfilling prophecies of poor outcomes (14). For patients with DOC who have a potential chance of consciousness recovery (6), skull defect is an unstable state of the brain, and CP is beneficial in reconstructing the cranial environment. CP can improve cerebral perfusion and brain function (1,2), and prevent or treat trephined syndrome/sinking skin flap syndrome (SSFS) caused by external atmospheric pressure in skull defect (15,16), in addition to protecting brain tissue, providing cosmetic benefits, and facilitating discharge home (17). The consciousness of patients was also reported to improve significantly after CP (18).
In our study, recovery of consciousness was observed in both the TBI and non-TBI groups. At the 1-year follow-up stage, 25 patients (28.7%) had a GOSE score of ≥3 and regained consciousness. There were 10 patients with a GOSE score of up to 4 who achieved partial self-care, which significantly reduced nursing challenges and living costs. These are very good results for patients who have been explicitly diagnosed with prolonged DOC. Since preoperative MCS status was a favorable factor for achieving GOSE ≥3 points, patients with MCS could benefit more from cranial repair.
Timing of surgery
This study found that late CP (>90 days) was an unfavorable factor for GOSE ≥3 at follow-up, which is likely due to the following two reasons. Firstly, patients diagnosed with DOC within 3 months may have a better natural prognosis, and secondly, early CP may provide benefits to consciousness recovery. Previous studies and systematic reviews have found that the benefits of improved nervous system function were greater with early (≤90 days) compared with late surgery (>90 days) (3,19), whereas the greatest improvement in cognitive function was noted when CP was performed within 3 to 6 months after DC (20).
The reported incidence of complications in early and late CP remains controversial. CP at 15 to 30 days may reduce the risks of infection, epilepsy, and bone flap absorption, whereas CP at >90 days minimizes the risk of hydrocephalus but increases the risk of epilepsy (5). A previous study on malignant middle cerebral artery infarction suggested that late CP after DC decreases the incidence of hydrocephalus (21). Incision complications may be frequent with early CP among patients with severe TBI having Glasgow Coma Scale scores of 3–8 points and bilateral frontotemporal bone window collapse after DC, whereas the long-term prognosis (according to the Glasgow Outcome Scale scores) may not differ between early and late CP (22). A meta-analysis found that early surgery can reduce the operation time and subdural effusion, and may be related to fewer scars (23). Delayed CP and the development of hydrocephalus are associated with poor prognosis in patients with severe TBI (17,24). In general, we believe that early surgery is safe and beneficial to the early improvement of consciousness and function, which is the premise of many rehabilitation programs and should be advocated.
Surgical complications
From the results of this study, CP was found to be safe for patients with DOC. Patients with DOC having skull defects often experience greater initial severe brain injury than conscious patients with skull defects. This includes damage to the cerebral vascular system, CSF circulatory system, and there may be hypothalamic insufficiency. The overall complication rate of CP was comparable to the rate reported in the literature (5). More extensive brain tissue damage in TBI patients may be related to the higher incidences of total complications compared to non-TBI patients. Traumatic subarachnoid hemorrhage in some TBI patients may also contribute to the high incidence of complications (25). Patients with DOC cannot actively express discomfort after surgery, which limits early detection of serious complications such as acute hydrocephalus and other secondary intracranial hypertension conditions. Therefore, targeted postoperative observation, such as routine lumbar puncture, is extremely important. Preoperative VPS is a risk factor for incision complications, which may be due to the poor wound conditions caused by a sunken skin flap. Preoperative VPS is also an important risk factor for epidural collection (26).
Management of TCH and hydrocephalus
There are two possible causes of postoperative hydrocephalus (as a main complication after CP) for patients with DOC. Firstly, severe brain injury significantly disrupts CSF circulation and increases the incidence of potential hydrocephalus (27). Secondly, the strategy to avoid preoperative shunt, as adopted in this study, makes the incidence of postoperative hydrocephalus appear to be high. Patients commonly show TCH, subdural effusion, and ventricular enlargement with DOC, and skull defects are related to extensive brain damage. Sometimes these issues occur concurrently, and transcalvarial brain herniation and contralateral or interhemispheric subdural hygroma may be a risk factor for post-traumatic hydrocephalus (13,28,29), which requires VPS placement (30).
However, the identification of the causes of ventricular enlargement, including hydrocephalus and brain atrophy, in patients with DOC is often difficult. This mirrors the dilemma of identifying normal-pressure hydrocephalus and brain atrophy in dementia patients. Thus, it is necessary to perform CSF drainage tests or assess CSF dynamics (31) before VPS placement. Improper VPS placement with skull defect, especially the use of a non-adjustable shunt, could not only increase hyperdrainage complications due to the atmospheric external pressure, but also cause problems for subsequent CPs, including epidural collection (26), lethal intracranial hypotension, and hypertension (32).
To avoid these risks, we strictly followed the indications for the procedure (as described in the Methods section), and performed CP soon after VPS placement. For the 45 patients with simple TCH, we performed various CSF release measures preoperatively to provide sufficient conditions for the brain tissue to return to its original form, and 36 (86.7%) of them turned out to be reversible. Although univariate analysis showed that irreversible TCH was significantly higher in the hydrocephalus group, regression analysis indicated that TBI, rather than TCH as a whole, was a risk factor for postoperative hydrocephalus. In some cases, it was necessary to wait for approximately 2 weeks after surgery to confirm that the intracranial pressure had gradually reduced to normal. This validates the theory that DC may have implications involving the flow of CSF (33). Other studies have found that CP could correct CSF disturbance (24). There is a need to further investigate whether delayed CP causes irreversible hydrocephalus. However, CP, rather than aggressive VPS, is the best treatment for patients with unstable states, such as TCH, and should be performed as early as possible. For potential hydrocephalus, a diagnosis and a decision on VPS can sometimes only be made after CP.
Nursing and rehabilitation
CP undoubtedly facilitates nursing and rehabilitation training, and may reduce the difficulty of discharge planning. In patients with DOC, general perioperative care, such as nutritional support, pulmonary care, management of severe cramp and diffuse pain, and rehabilitation exercise for limb function, should also be emphasized; although, data on the well-known efficacy of neurorehabilitation in neurosurgical patients is scarce (17). Attention should also be paid to the management of comorbidities and medical complications, which may be critical in influencing the recovery of consciousness and functional improvement (34,35).
Limitations
The limitations of this study include the following. Firstly, the retrospective collection and analysis of the data cannot disentangle the effect of CP from natural recovery in the improvement of the level of consciousness. Secondly, due to the disability and transportation difficulties of patients with DOC, bedside evaluations of CRS-R were replaced by the use of GOSE (via telephone) in the follow-up assessment, which had a limited correlation with CRS-R used in the preoperative evaluation. A more recommended GOSE-Revised (GOSE-R) (36,37) will be used in future studies. Thirdly, intracranial pressure measured through lumbar puncture was influenced by deviations in patient posture and state [e.g., if a patient was in paroxysmal sympathetic hyperactivity state (38), a common syndrome in patients with DOC appearing hypertonia or spasticity, the pressure would increase accordingly]. Special attention to the measurement environment was required, and multiple measurements were conducted to avoid single measurement bias. Lastly, electroencephalography was not performed during the perioperative period to protect the wound, and it is difficult to diagnose epilepsy using the clinical manifestations of patients with DOC. Therefore, we did not consider epilepsy as a surgical complication, which might have influenced the estimation of total complications.
Conclusions
CP is beneficial in DOC patients with severe brain injury. Although the complications and costs of TBI patients were higher than those of non-TBI patients, there was no significant difference in the outcomes. However, early surgery and surgery for patients with a MCS diagnosis resulted in better consciousness recovery. Preoperative VPS was a risk factor for postoperative incision complications, and non-traumatic etiology tended to be a protective factor against postoperative hydrocephalus. For patients with simple TCH, a decision on VPS can only be made after CP. Special observations, such as routine measurement of intracranial pressure and neurorehabilitation for DOC patients, are essential for ensuring optimal prognosis.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (No. 81771128).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/apm-21-1822
Data Sharing Statement: Available at https://dx.doi.org/10.21037/apm-21-1822
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apm-21-1822). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee of The Seventh Medical Center, Chinese PLA General Hospital (approval number 2019-061), and informed consent was obtained from all patients’ surrogates. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Halani SH, Chu JK, Malcolm JG, et al. Effects of cranioplasty on cerebral blood flow following decompressive craniectomy: a systematic review of the literature. Neurosurgery 2017;81:204-16. [Crossref] [PubMed]
- Shahid AH, Mohanty M, Singla N, et al. The effect of cranioplasty following decompressive craniectomy on cerebral blood perfusion, neurological, and cognitive outcome. J Neurosurg 2018;128:229-35. [Crossref] [PubMed]
- Malcolm JG, Rindler RS, Chu JK, et al. Early cranioplasty is associated with greater neurological improvement: a systematic review and meta-analysis. Neurosurgery 2018;82:278-88. [Crossref] [PubMed]
- Yang NR, Song J, Yoon KW, et al. How early can we perform cranioplasty for traumatic brain injury after decompressive craniectomy? A retrospective multicenter study. World Neurosurg 2018;110:e160-7. [Crossref] [PubMed]
- Morton RP, Abecassis IJ, Hanson JF, et al. Timing of cranioplasty: a 10.75-year single-center analysis of 754 patients. J Neurosurg 2018;128:1648-52. [Crossref] [PubMed]
- Estraneo A, Fiorenza S, Magliacano A, et al. Multicenter prospective study on predictors of short-term outcome in disorders of consciousness. Neurology 2020;95:e1488-99. [Crossref] [PubMed]
- Missori P, Miscusi M, Formisano R, et al. Magnetic resonance imaging flow void changes after cerebrospinal fluid shunt in post-traumatic hydrocephalus: clinical correlations and outcome. Neurosurg Rev 2006;29:224-8. [Crossref] [PubMed]
- Formisano R, Giustini M, Aloisi M, et al. An international survey on diagnostic and prognostic protocols in patients with disorder of consciousness. Brain Inj 2019;33:974-84. [Crossref] [PubMed]
- Laureys S, Celesia GG, Cohadon F, et al. Unresponsive wakefulness syndrome: a new name for the vegetative state or apallic syndrome. BMC Med 2010;8:68. [Crossref] [PubMed]
- Kaushal A, Bindra A, Kumar A, et al. Long term outcome in survivors of decompressive craniectomy following severe traumatic brain injury. Asian J Neurosurg 2019;14:52-7. [Crossref] [PubMed]
- Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma 1998;15:573-85. [Crossref] [PubMed]
- Stiver SI. Complications of decompressive craniectomy for traumatic brain injury. Neurosurg Focus 2009;26:E7 [Crossref] [PubMed]
- Vedantam A, Yamal JM, Hwang H, et al. Factors associated with shunt-dependent hydrocephalus after decompressive craniectomy for traumatic brain injury. J Neurosurg 2018;128:1547-52. [Crossref] [PubMed]
- Septien S, Rubin MA. Disorders of consciousness: ethical issues of diagnosis, treatment, and prognostication. Semin Neurol 2018;38:548-54. [Crossref] [PubMed]
- Han PY, Kim JH, Kang HI, et al. "Syndrome of the sinking skin-flap" secondary to the ventriculoperitoneal shunt after craniectomy. J Korean Neurosurg Soc 2008;43:51-3. [Crossref] [PubMed]
- Ashayeri K, Jackson EM, Huang J, et al. Syndrome of the trephined: a systematic review. Neurosurgery 2016;79:525-34. [Crossref] [PubMed]
- Formisano R, Contrada M, Colli G, et al. Decompressive craniectomy and cranioplasty: the point of view of the neurorehabilitation team. J Neurosurg Sci 2020;64:494-5. [Crossref] [PubMed]
- Corallo F, Calabro RS, Leo A, et al. Can cranioplasty be effective in improving cognitive and motor function in patients with chronic disorders of consciousness? A case report. Turk Neurosurg 2015;25:193-6. [PubMed]
- Bjornson A, Tajsic T, Kolias AG, et al. A case series of early and late cranioplasty-comparison of surgical outcomes. Acta Neurochir (Wien) 2019;161:467-72. [Crossref] [PubMed]
- De Cola MC, Corallo F, Pria D, et al. Timing for cranioplasty to improve neurological outcome: a systematic review. Brain Behav 2018;8:e01106 [Crossref] [PubMed]
- Finger T, Prinz V, Schreck E, et al. Impact of timing of cranioplasty on hydrocephalus after decompressive hemicraniectomy in malignant middle cerebral artery infarction. Clin Neurol Neurosurg 2017;153:27-34. [Crossref] [PubMed]
- Zhu H, Ji C, Shen Z, et al. Early cranioplasty benefits patients with obvious bilateral frontotemporal bone window collapse after decompressive craniectomy. World Neurosurg 2018;113:198-203. [Crossref] [PubMed]
- Zheng F, Xu H, von Spreckelsen N, et al. Early or late cranioplasty following decompressive craniotomy for traumatic brain injury: a systematic review and meta-analysis. J Int Med Res 2018;46:2503-12. [Crossref] [PubMed]
- Nasi D, Dobran M, Di RA, et al. Decompressive craniectomy for traumatic brain injury: the role of cranioplasty and hydrocephalus on outcome. World Neurosurg 2018;116:e543-9. [Crossref] [PubMed]
- Posti JP, Yli-Olli M, Heiskanen L, et al. Cranioplasty after severe traumatic brain injury: effects of trauma and patient recovery on cranioplasty outcome. Front Neurol 2018;9:223. [Crossref] [PubMed]
- Zheng WJ, Li LM, Hu ZH, et al. Complications in staged late titanium cranioplasty and ventriculoperitoneal shunting for patients with traumatic brain injury. World Neurosurg 2019;127:e1166-71. [Crossref] [PubMed]
- Chen H, Yuan F, Chen SW, et al. Predicting posttraumatic hydrocephalus: derivation and validation of a risk scoring system based on clinical characteristics. Metab Brain Dis 2017;32:1427-35. [Crossref] [PubMed]
- Silva Neto AR, Valença MM. Transcalvarial brain herniation volume as a predictor of posttraumatic hydrocephalus after decompressive craniectomy. Clin Neurol Neurosurg 2019;182:73-8. [Crossref] [PubMed]
- Su TM, Lan CM, Lee TH, et al. Risk factors for the development of posttraumatic hydrocephalus after unilateral decompressive craniectomy in patients with traumatic brain injury. J Clin Neurosci 2019;63:62-7. [Crossref] [PubMed]
- Wu R, Ye Y, Ma T, et al. Management of subdural effusion and hydrocephalus following decompressive craniectomy for posttraumatic cerebral infarction in a patient with traumatic brain injury: a case report. BMC Surg 2019;19:26. [Crossref] [PubMed]
- Marmarou A, Foda MA, Bandoh K, et al. Posttraumatic ventriculomegaly: hydrocephalus or atrophy? A new approach for diagnosis using CSF dynamics. J Neurosurg 1996;85:1026-35. [Crossref] [PubMed]
- Dalle Ore CL, Abraham P, Burns LP, et al. Intracranial hypotension and hypertension associated with reconstructive cranioplasty after decompressive craniectomy: report of a lethal complication with recommended strategies for future avoidance. J Craniofac Surg 2018;29:1862-4. [Crossref] [PubMed]
- Lilja-Cyron A, Andresen M, Kelsen J, et al. Long-term effect of decompressive craniectomy on intracranial pressure and possible implications for intracranial fluid movements. Neurosurgery 2020;86:231-40. [PubMed]
- Pistoia F, Sacco S, Franceschini M, et al. Comorbidities: a key issue in patients with disorders of consciousness. J Neurotrauma 2015;32:682-8. [Crossref] [PubMed]
- Estraneo A, Loreto V, Masotta O, et al. Do medical complications impact long-term outcomes in prolonged disorders of consciousness. Arch Phys Med Rehabil 2018;99:2523-31.e3. [Crossref] [PubMed]
- Formisano R, Aloisi M, Ferri G, et al. The Glasgow Outcome Scale Extended-Revised (GOSE-R) to include minimally conscious state in the vegetative state category. J Neurol Sci 2018;388:22. [Crossref] [PubMed]
- Formisano R, Contrada M, Ferri G, et al. The Glasgow Outcome Scale Extended-Revised (GOSE-R) to include minimally conscious state in the vegetative state/unresponsive wakefulness syndrome category: a correlation with Coma Recovery Scale-Revised (CRS-R). Eur J Phys Rehabil Med 2019;55:139-40. [Crossref] [PubMed]
- Zheng RZ, Lei ZQ, Yang RZ, et al. Identification and management of paroxysmal sympathetic hyperactivity after traumatic brain injury. Front Neurol 2020;11:81. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


