
Pathogen profile and risk factors of aerobic vaginitis in pregnant women: a retrospective cohort study
Introduction
Vaginitis is a common gynecological disease in women of all ages (1), the prevalance of which varies with living habits, living environment, religious beliefs, and economic status. Several pathogens may cause the disease, and may take the form of bacterial vaginitis, mixed vaginitis, Candida vaginitis, Mycoplasma vaginitis, and Trichomonas vaginitis (2), the latter having been the most common type in past decades (3). However, the incidence of Trichomonas vaginitis has been decreasing in recent years, while that of bacterial vaginitis has remained stable (4). Aerobic vaginitis (AV) is more common than anaerobic vaginitis among all bacterial vaginitis (5), and the pathogens of AV include aerobic microorganisms from the gastrointestinal tract such as Escherichia coli, staphylococcus aureus, coagulase-negative staphylococci and group B streptococcus (6,7). Patients with AV may suffer from abnormal vaginal discharge and vulvae itching and discomfort, which severely affect their quality of life.
During pregnancy, women will experience various hormonal and physiological changes, which also include changes in the vaginal environment. This may lead to an increased incidence rate of vaginitis and changes to pathogens (8). It has been reported that the pathogen profile of AV in late pregnancy differed from that in non-pregnant women in a Chinese population, with more group B streptococcus and less coagulase-negative staphylococci and staphylococcus aureus (9). The occurrence of AV may worsen the outcomes of pregnant women, resulting in more spontaneous preterm delivery and premature rupture of membranes (9-11). However, previous studies mainly focused on early or late stage pregnancy, and it is hard to understand the incidence of vaginitis across the whole of pregnancy. The study of risk factors of vaginitis in pregnant women also has important clinical significance. Many studies have explored the risk factors of vaginitis in non-pregnant women (12-14), which may differ significantly from those of non-pregnant women, due to hormonal and physiological changes. However, few studies have explored this issue to date, and the risk factors of AV in pregnant women remain unclear.
This study was designed to determine the prevalence and pathogen profile of AV in women at all stages of pregnancy and investigate the effects of AV on pregnancy outcomes. Most importantly, this study aimed to explore the risk factors of AV in pregnant women. The results may assist pregnant women reduce the incidence of AV and improve pregnancy outcomes. We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/apm-21-1710).
Methods
Participant enrollment
We retrospectively enrolled pregnant women who were admitted to the Wuxi Maternity and Child Health Care Hospital of Nanjing Medical University between July 2018 and June 2020, and divided them into a group with AV and a healthy control group. Women older than 18 years old and those who constantly received prenatal examination in our hospital were included, while those who were diagnosed with vaginitis other than AV or mixed vaginitis, did not attend for regular prenatal examinations and were lost to follow up, who received long term antibiotic treatment, who needed to terminate their pregnancy because of severe complications, were excluded. This study was approved by the Ethics Committee of the Affiliated Wuxi Maternity and Child Health Care Hospital of Nanjing Medical University (No. 20200037) and was conducted in compliance with the ethical principles of the Declaration of Helsinki (as revised in 2013). Written informed consent was not required due to the retrospective nature of the study.
Data collection
Following enrolment, the baseline characteristics of patients was collected including age, body mass index, multifetal pregnancy, parity, history of cesarean delivery, history of vaginal infection, history of hypertension and diabetes mellitus, level of education, history of smoking, and occupational status. The data were collected by two independent investigators to minimize errors.
All enrolled participants underwent a gynecological examination, and a sterile cotton swab was used to obtain samples of vaginal discharge. The diagnosis of AV was determined by lactobacillary grade, leukocyte number, proportion of toxic leukocytes, background flora, and proportion of parabasal epitheliocytes according to microscopic examination. Pathogens of AV were tested by conventional bacterial culture and biochemical analysis. Part pathogens were tested using quantitative real-time polymerase chain reaction for amplification of specific targets, followed by specific probe test for bacterial vaginosis markers, including Enterococcus group, bacterial vaginosis-associated bacterium 2, and Megasphaera 1. The gestational week of participants when diagnosed with AV was also recorded.
Pregnancy outcomes
All participants were followed up until 1 month after delivery. Some pregnant outcomes were recorded in this study for further analysis, including delivery mode, preterm birth, premature rupture of membranes, birth weight, Apgar score, neonatal jaundice, neonatal infection, and stillbirth.
Statistical analyses
Continuous variables were shown as mean with standard deviation and categorical variables were shown as number with percentage. Comparisons between continuous variables were analyzed using student t-test and those between categorical variables using Chi-square test. Univariable logistic regression analysis was performed to preliminarily determine the risk factors of adverse pregnant outcomes and AV. Multivariable logistic regression analysis was then used to verify the real risk factors after adjusting confounding variables according to univariable logistic regression analysis. Statistical analysis was performed using SPSS 20.0 (IBM Corporation, NY, USA) and P value less than 0.05 was considered statistically significant.
Results
We enrolled pregnant women who had attended our hospital over a 2-year period and the flow chart is shown in Figure 1. In total, 1,296 women were admitted and 685 were enrolled in the study after 611 women failed to meet the exclusion criteria. Those included in the study were then divided into an AV group of 182 women and a healthy control group of 503.
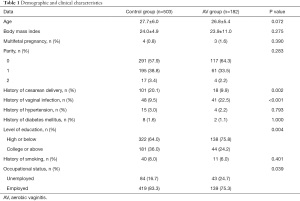
Demographic and clinical characteristics of both groups are shown in Table 1. In the control group, the mean age was 27.7±6.0 years and mean body mass index was 24.0±4.9. Four women had multifetal pregnancies, and more than half of the control group were primiparas. A history of cesarean delivery was seen in 101 women, 48 women had a history of vaginal infection, 40 had a history of smoking, 15 had hypertension, and eight women had diabetes. High school education or below was recorded in 322 women in the control group, and 419 were currently employed.
Full table
In the AV group, the mean age was 26.8±5.4 years and mean body mass index was 23.9±11.0. Three women had multifetal pregnancies and 117 women were primiparas. A history of cesarean delivery was seen in 18 women, 41 women had a history of vaginal infection, 11 had a history of smoking before pregnancy, four had hypertension, and two had diabetes mellitus. Around 75% of women in the AV group had received high school education or below, and 139 women were employed.
Comparison of the demographic and clinical characteristics in the AV group and control group showed significantly more women in the control group had a history of cesarean delivery (20.1% vs. 9.9%, P=0.002), while 22.5% of the AV group had a history of vaginal infection, which was much more than the 9.5% in the control group (P<0.001). More women in the AV group received high school education or below compared to the control group (75.8% vs. 64.0%, P=0.004), and the proportion of unemployed women in the AV group was higher than in the control group (24.7% vs. 16.7%, P=0.039).
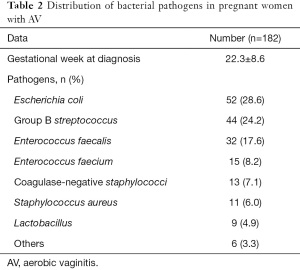
The distribution of bacterial pathogens in pregnant women with AV is summarized in Table 2. The mean gestational week at diagnosis of AV was 22.3±8.6 weeks. The most common pathogen was Escherichia coli (28.6%), followed by group B streptococcus (24.2%), Enterococcus faecalis (17.6%), Enterococcus faecium (8.2%), coagulase-negative staphylococci (7.1%), staphylococcus aureus (6.0%), lactobacillus (4.9%), and other pathogens (3.3%).
Full table
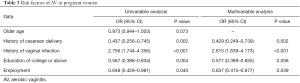
Risk factors of AV were analyzed using logistic regression as shown in Table 3. Older age, history of cesarean delivery, history of vaginal infection, college education or above, and employment showed significant effects on the incidence of AV according to univariable logistic regression. However, after adjustment using multivariable logistic regression, a history of vaginal infection acted as an important risk factor of AV incidence, and a history of cesarean delivery, college education or above, and employment could protect pregnant women from the incidence of AV.
Full table
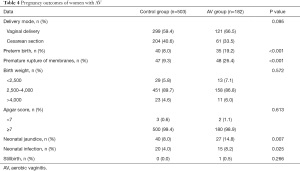
Pregnancy outcomes of pregnant women with AV in both groups are also listed in Table 4. Vaginal delivery took place in 299 and 121 women in the control group and AV group, respectively, and preterm birth occurred in 40 women (8.0%) in the control group and 35 women (19.2%) in the AV group, showing significant difference (P<0.001). Similarly, premature rupture of membranes occurred in 47 women (9.3%) in the control group and 48 women (26.4%) in the AV group, showing significant difference (P<0.001). Neonatal jaundice was observed in 40 neonates (8.0%) of the control group and 27 neonates (14.8%) of the AV group, showing a significant difference (P=0.007), and neonatal infection was observed in 20 neonates (4.0%) of the control group and 15 (8.2%) of the AV group, also showing a significant difference (P=0.025). Only one stillbirth was observed in all enrolled women of both groups.
Full table
Finally, the risk factors of premature rupture of membranes and neonatal infection were also analyzed and are shown in Table 5. After adjustment using multivariable logistic regression, the incidence of AV, older age, and higher body mass index were identified as important risk factors of premature rupture of membranes. Similarly, the incidence of AV and older age were also important risk factors of neonatal infection.

Full table
Discussion
This retrospective cohort study enrolled 685 pregnant women from a single center. We measured the pathogen profile of AV in these pregnancies as well as the gestational week at diagnosis of AV. The risk factors of AV were then identified using multivariable logistic regression, and included history of vaginal infection, history of cesarean delivery, college education or above, and being unemployed. The results of this study may assist in the treatment and prevention of AV in pregnant women.
In recent years, Trichomonas vaginitis has been decreasing while the incidence rate of AV has remained stable. A total of 665 pregnant women in this study suffered from vaginitis, of which 182 had AV, accounting for 27.3%. The proportion of AV was slightly higher than that in previous studies, which ranged from 4.2% to 23.7% (10,12,15,16). Krauss-Silva et al. reported a prevalence of 32.5% of bacterial vaginitis in black women and 28.1% in white women, indicating the living environment and race could significantly affect the incidence of bacterial vaginitis (16). Furthermore, some previous studies divided AV patients into a slight AV group and severe group, which may provide more detailed analysis for its risk factors. However, it was difficult to group participants according to the severity of AV in this study due to its retrospective nature. Escherichia coli was the most common pathogen of AV in our study, which is consistent with the results of previous studies (10,13,17). This also reveals that one of the main sources of vaginitis is the gastrointestinal tract. It has been reported that both early and late pregnancy had a higher incidence rate of AV (9,10,18,19). The mean gestational week at diagnosis of AV in our study was 22.3±8.6 weeks, which may show that there is no special gestational age with high risk for AV in pregnant women.
We then determined the risk factors of AV according to multivariable logistic regression and found the most important risk factor to be a history of vaginal infection, which increased the risk of AV by 2.6 times and P<0.001. This result aligns with that of Han et al., who reported a history of vaginal infection within 1 year would increase the risk of AV by 3.2 times in pregnant women (10). Previous studies have shown intrauterine device use, external hemorrhoids, long-term antibiotic use, and frequent vaginal douching were independent risk factors for AV (10,13). We found higher educational level and being employed may also help reduce the incidence of AV in pregnant women, which is also similar to previous studies (20,21). Interestingly, we found that a history of cesarean delivery may play a protective role in pregnant women with AV. This may be because vaginal delivery can lead to changes in the vaginal flora and slight structural damage, resulting in the increased risk of AV in future pregnancies. We found that the presence of AV worsened pregnancy outcomes, by increasing the incidence of preterm birth, the premature rupture of membranes, neonatal jaundice, and neonatal infection. A Chinese population study also confirmed that AV would increase the incidence of neonatal jaundice and neonatal infection (9), and a systematic review of 12 related studies showed an association between AV and preterm birth and premature rupture of membranes (8). However, it also found that AV would increase the proportion of neonates with low birth weight. Only 42 neonates with low birth weight were delivered in our study and there was no significant difference between the two groups. After adjustment using multivariable logistic regression, older age, especially older than 35 years, was also seen as an important risk factor of adverse pregnant outcomes, which is similar to the results of a previous study (22).
There are some limitations in our study. Firstly, as a retrospective study, the types of data that can be collected are relatively limited. Some other data such as the results of serological and immunological examinations could not be obtained, which may affect the final result. Secondly, follow-up and data collection were performed when women attended hospital for prenatal examination and delivery, and some risk factors of AV and adverse pregnancy outcomes that may have existed out of hospital and after delivery could also not be collected. Thirdly, different pathogens of AV were identified in our study, and it is still unclear what effect each of these pathogens has on pregnancy outcomes. Studies focusing on the effect of a single pathogen may improve the quality of future studies.
Our study enrolled 685 pregnant women including 503 healthy women and 182 with AV and found that the incidence of AV increased the incidence of adverse pregnancy outcomes. To reduce the incidence of AV in pregnancy, more attention should be paid to women with a history of vaginal infection. The results from our study provide some evidence for clinical care and treatment in pregnancy.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/apm-21-1710
Data Sharing Statement: Available at https://dx.doi.org/10.21037/apm-21-1710
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apm-21-1710). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee of the Affiliated Wuxi Maternity and Child Health Care Hospital of Nanjing Medical University (No. 20200037) and was conducted in compliance with the ethical principles of the Declaration of Helsinki (as revised in 2013). Written informed consent was not required due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sonthalia S, Aggarwal P, Das S, et al. Aerobic vaginitis - An underdiagnosed cause of vaginal discharge - Narrative review. Int J STD AIDS 2020;31:1018-27. [Crossref] [PubMed]
- Rigo GV, Tasca T. Vaginitis: review on drug resistance. Curr Drug Targets 2020;21:1672-86. [Crossref] [PubMed]
- Han C, Wu W, Fan A, et al. Diagnostic and therapeutic advancements for aerobic vaginitis. Arch Gynecol Obstet 2015;291:251-7. [Crossref] [PubMed]
- Abdul-Aziz M, Mahdy MAK, Abdul-Ghani R, et al. Bacterial vaginosis, vulvovaginal candidiasis and trichomonal vaginitis among reproductive-aged women seeking primary healthcare in Sana'a city, Yemen. BMC Infect Dis 2019;19:879. [Crossref] [PubMed]
- Tao Z, Zhang L, Zhang Q, et al. The pathogenesis of streptococcus anginosus in aerobic vaginitis. Infect Drug Resist 2019;12:3745-54. [Crossref] [PubMed]
- Zhang HT, Wang H, Wu HS, et al. Comparison of viromes in vaginal secretion from pregnant women with and without vaginitis. Virol J 2021;18:11. [Crossref] [PubMed]
- Lin Z, Lin Y, Zhang Z, et al. Systematic analysis of bacteriostatic mechanism of flavonoids using transcriptome and its therapeutic effect on vaginitis. Aging 2020;12:6292-305. [Crossref] [PubMed]
- Juliana NCA, Suiters MJM, Al-Nasiry S, et al. The association between vaginal microbiota dysbiosis, bacterial vaginosis, and aerobic vaginitis, and adverse pregnancy outcomes of women living in Sub-Saharan Africa: a systematic review. Front Public Health 2020;8:567885 [Crossref] [PubMed]
- Tang Y, Yu F, Hu Z, et al. Characterization of aerobic vaginitis in late pregnancy in a Chinese population: a STROBE-compliant study. Medicine (Baltimore) 2020;99:e20732 [Crossref] [PubMed]
- Han C, Li H, Han L, et al. Aerobic vaginitis in late pregnancy and outcomes of pregnancy. Eur J Clin Microbiol Infect Dis 2019;38:233-9. [Crossref] [PubMed]
- Novakov Mikić A, Stojic S. Study results on the use of different therapies for the treatment of vaginitis in hospitalised pregnant women. Arch Gynecol Obstet 2015;292:371-6. [Crossref] [PubMed]
- Wang H, Huang Z, Wu Z, et al. An epidemiological study on vaginitis in 6,150 women of reproductive age in Shanghai. New Microbiol 2017;40:113-8. [PubMed]
- Geng N, Wu W, Fan A, et al. Analysis of the risk factors for aerobic vaginitis: a case-control study. Gynecol Obstet Invest 2015; Epub ahead of print. [Crossref] [PubMed]
- Pek E, Beyazit F, Korkmaz NS. Predictive value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with vaginitis. Pak J Med Sci 2021;37:250-5. [PubMed]
- Rumyantseva TA, Bellen G, Savochkina YA, et al. Diagnosis of aerobic vaginitis by quantitative real-time PCR. Arch Gynecol Obstet 2016;294:109-14. [Crossref] [PubMed]
- Krauss-Silva L, Almada-Horta A, Alves MB, et al. Basic vaginal pH, bacterial vaginosis and aerobic vaginitis: prevalence in early pregnancy and risk of spontaneous preterm delivery, a prospective study in a low socioeconomic and multiethnic South American population. BMC Pregnancy Childbirth 2014;14:107. [Crossref] [PubMed]
- Lynch T, Peirano G, Lloyd T, et al. Molecular diagnosis of vaginitis: comparing quantitative PCR and microbiome profiling approaches to current microscopy scoring. J Clin Microbiol 2019;57:e00300-19. [Crossref] [PubMed]
- Masukume G, Khashan AS, Kenny LC, et al. Risk factors and birth outcomes of anaemia in early pregnancy in a nulliparous cohort. PLoS One 2015;10:e0122729 [Crossref] [PubMed]
- Du M, Liu J, Han N, et al. Maternal sleep quality during early pregnancy, risk factors and its impact on pregnancy outcomes: a prospective cohort study. Sleep Med 2021;79:11-8. [Crossref] [PubMed]
- Schwebke JR, Gaydos CA, Nyirjesy P, et al. Diagnostic performance of a molecular test versus clinician assessment of vaginitis. J Clin Microbiol 2018;56:e00252-18. [Crossref] [PubMed]
- Xu F, Du X, Xie L. Vaginitis in pregnancy is related to adverse perinatal outcome. Pak J Med Sci 2015;31:582-6. [PubMed]
- Xie D, Xiang Y, Wang A, et al. The risk factors of adverse pregnancy outcome for pre-pregnancy couples in Hunan, China: a cross-sectional study based on population. Medicine (Baltimore) 2020;99:e23094 [Crossref] [PubMed]
(English Language Editor: B. Draper)



