
Systematic review and meta-analysis of the sedative effects and safety of dexmedetomidine in patients after cardiac surgery
Introduction
Sedation and analgesia both play important roles in the treatment of critically ill patients. Moderate sedation in patients receiving mechanical ventilation can effectively alleviate discomfort, eliminate anxiety, reduce the stress response, increase tolerance to endotracheal tubes and mechanical ventilation, aid and improve sleep, and induce amnesic effects. Furthermore, pain during treatment reduces a patient’s metabolic rate and oxygen consumption (1,2), and improper sedation may put a critically ill patient at risk. For example, undersedation can cause restlessness, increased oxygen demand, reduced human-machine coordination, and accidental extubation, while oversedation can cause disordered circulatory and respiratory functions, delayed weaning, a prolonged intensive care unit (ICU) stay, and an increased incidence of ventilator associated pneumonia (VAP) (3,4).
Dexmedetomidine is a highly selective alpha-2 (α2) adrenergic receptor agonist, which produces corresponding pharmacological effects by acting on α2 receptors in both the central and peripheral nervous systems (5,6). Dexmedetomidine produces sedative and hypnotic effects by acting on locus coeruleus α2 receptors and activating endogenous sleep-promoting pathways, which allows patients to maintain non-rapid eye movement stage 3 sleep. The main characteristic of this sedative-hypnotic state is that a patient can be awakened by stimulation or language, and there is also no accompanying respiratory depression (7,8). Dexmedetomidine has the benefits of decreasing anxiety, reducing the stress response, stabilizing hemodynamics, inducing analgesia, inhibiting salivary gland secretion, combating rigors, and managing diuresis (9,10). In addition, dexmedetomidine has useful sedative effects when combined with other sedative and analgesic drugs, which can greatly reduce the need for other medications (11,12).
Dexmedetomidine can be administered by intravenous pump, intramuscular injection, intranasal drip, or buccal, mucosal, or oral administration. It has a liver first-pass elimination effect, with an oral bioavailability of 16% (13), and the plasma clearance rate of dexmedetomidine decreases as the severity of liver damage increases. The dose of dexmedetomidine can be reduced as appropriate for patients with liver damage, and patients with renal dysfunction generally do not require an adjusted dose (14). The clinical selection of analgesic and sedative drugs needs to meet the following characteristics: small effect on circulation and respiration, rapid onset and significant effect, rapid drug metabolism and small effect on liver and kidney function. At present, the commonly used drugs include benzodiazepines, propofol, α2 receptor agonist (dexmedetomidine), and studies have found that dexmedetomidine has a positive effect in reducing sympathetic tension, lowering blood pressure and slowing heart rate, Meta. Therefore, meta-analysis was used in this study to study the sedative effects and safety of dexmedetomidine on patients after cardiac surgery. We present the following article in accordance with the PRISMA reporting checklist (available at https://dx.doi.org/10.21037/apm-21-1850).
Methods
Literature retrieval
Relevant documents were selected using the Boolean search method, with “dexmedetomidine”, “cardiac surgery”, “competitive antagonist”, and “analgesic sedation”, used as keywords during searches of PubMed, Medline, Embase, China Biomedical Literature Database, HowNet, Wanfang Database, Weipu Database, and Google Scholar. All literature was examined to find articles yet to be indexed by the database literature. The retrieval time is from the establishment of the database to May 30, 2021. According to RevMan 5.2 software provided by Cochrane system, the literature quality was evaluated. All kinds of search words are freely combined, and after searching for certain documents for many times, the search engine is used to trace the certain documents. And get the latest research progress after contacting experts and researchers in the field.
Inclusion and exclusion criteria
The included literatures should meet all the following criteria: (I) the subjects are patients undergoing severe cardiac surgery; (II) intervention measures for patients: Dexmedetomidine was used as sedative in the experimental group. (III) Midazolam/propofol was used as sedative in the control group. (IV) The evaluation indexes were sedation before and after operation, postoperative nausea and vomiting, recovery time, etc.
The literatures that meet any of the following criteria are excluded: (I) the included subjects have hemodynamic instability, cardiac dysfunction and central nervous system dysfunction; (II) the research types are retrospective studies, cohort studies, case reports and other non-RCT studies; (III) the included research objects are accompanied by mental diseases or infectious diseases; (IV) no valid data is provided or literature is missing; (V) the research objects or data overlap with each other. Two senior experts were required to independently screen the title, abstract, and full text of each study, and 3 preliminary experiments were completed before screening commenced. Any inconsistencies were solved by discussion between the 2 experts or arbitration by a third expert.
Quality evaluation
In this study, Cochrane Handbook5.0.2 is used to evaluate the bias risk of 12 papers included in this study, and the evaluation results are input into Review Manager5.3 software to generate the bias risk map. A star rating system (out of 9 stars) was used to measure the results from subjects, case comparison and comparison between groups. The selected literature with 7 stars or above can be considered as high quality, that is, low risk bias; References in 1 star or no star can be considered as low quality, that is, (high risk bias); References in 2–6 stars can be considered as medium quality, that is, (medium risk bias). Two experts were required for quality assessment of the literature, and three experiments had to be conducted before screening. When two experts disagree, a discussion is needed to solve the problem.
Data extraction
The data were independently extracted using an Excel spreadsheet (Microsoft Corporation), and 3 experiments had to be performed before the extraction could commence. If there was any inconsistency, it was solved by discussion between the 2 experts or by arbitration. The data included the following: the study’s first listed author and its year of publication; the number of patients in the study; the grouping of the patients and the postoperative sedation methods used for group A and group B; and parameters and indicators on the level of sedation after cardiac surgery, such as mechanical ventilation time (MVT), length of stay, complications, hemodynamic indicators, and sedative effect during induction of anesthesia.
Statistical methods
The meta-analysis was conducted using Review Manager 5.3 software (Cochrane). The included studies were tested for heterogeneity, and mean differences (MD) or standardized MD (SMD) and the 95% confidence interval (CI) were used to test the efficacy of the statistical analysis. The bias risk assessment chart from the Review Manager software was applied to assess the risk bias, so when P>0.1 and I2<50% or P<0.1 and I2>50%, a fixed effects model (FEM) or a random effects model (REM) was employed.
Results
Literature collection and NOS scale rating
As shown in Figure 1, there were 250 articles searched, with 140 articles being removed after the reading of abstracts and titles and 97 being removed after a reading of the full texts. In total, 13 articles were obtained for use in our study. The excluded literature included 48 articles whose patients had not undergone cardiac surgery or had other types of system diseases, 19 articles whose research involved animal experimentation, 39 articles with repeated research participants, 63 articles with unsuccessful data extraction, 51 articles taking nonhemodynamic parameters as research indicators, and 17 articles lacking original data for the research results. The basic information from the chosen literature is shown in Table 1, including the publication date of each article (between 2003 and 2013). Figure 2 shows the results of the NOS scale rating, revealing 4 selected articles with ≥7 stars, 9 articles with 2–6 stars, and 0 articles with <2 stars. Therefore, the articles included in our study were judged to be of medium and high quality.
Full table
Risk bias of articles
Figures 3 and 4 show the multiple risk bias evaluations, including random sequence generation, allocation hiding, blinding of result evaluation, and incomplete result data. All selected articles had a low bias level, and the blinding of the participants and researchers and other biases were about 50%. All articles except for those by Kevin et al. [2010] and Ghali et al. [2011] had a low-risk bias.
Comparison of MVT
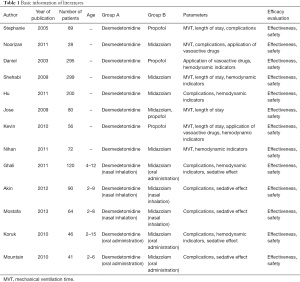
The MVT from the patients in group A and group B were compared, as shown in Figure 5, with the patients described by Stephanie et al. [2005] accounting for the largest percentage (16.7%), followed by the patients of Akin et al. [2012] (15.4%) and Nihan et al. [2011] (14.8%). The horizontal line (HL) of the 95% CI in most studies is on the left side of the invalid vertical line (IVL), with a small number of studies seen on the right side. Group A included 347 patients, while group B and 355 patients in, with no statistical heterogeneity in MVT between the groups being found (Chi2=74.71; I2=92%; P<0.00001). The combined effect size (CES; represented by diamond blocks in Figure 5) crossed the IVL, producing an odds ratio (OR) value of –2.28 and a 95% CI of –5.13 to 0.57. The random model analysis indicated an observable difference between the MVT of the 2 patients’ groups (Z=1.57; P=0.12).
Figure 6 presents a funnel chart comparing the MVT of patients from the 2 groups. The concentration of circles in the top area indicates the high accuracy of the included studies. Although the chart’s circles are seen on both sides of the midline, their asymmetry is indicative of the publication bias of an included study.
Comparison of patient length of stay
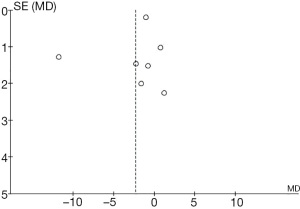
As illustrated in Figure 7, comparison and analysis of the 2 groups was carried out on the patients’ length of stay after cardiac surgery, with Stephanie’s et al. [2005] study accounting for the highest percentage of included patients (49.4%), followed by that of Shehabi et al. [2009] (42.6%). The HL of the 95% CI in most studies falls on the left side of the IVL, with the line falling on the right side in a small number of studies. Among the included studies, group A comprised 299 patients and group B 303 patients, with statistical heterogeneity visible in the patient length of stay (Chi2=14.62; I2=73%; P=0.006). The CES is seen to the left of the IVL, with an OR value of –1.24 and a 95% CI of –4.35 to 1.87). The random model analysis shows no evidence of any remarkable differences in patient length of stay between the groups (Z=0.78; P=0.43).
Figure 8 is a funnel chart comparing the length of stay between the 2 groups of patients after cardiac surgery. The circles are distributed on both sides but are not symmetrical, indicating the existence of publication bias.
Comparison of incidence of complications
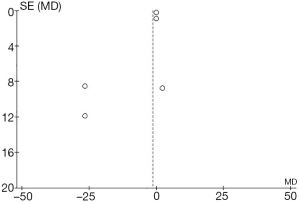
Comparison results of postoperative complications are illustrated in Figure 9. The most common complications were bradycardia, ventricular tachycardia, restlessness, nausea and vomiting, postoperative hyperglycemia, heart failure, myocardial infarction, and acute exhaustion. Research results from Shehabi et al. [2009] accounted for the highest percentage (24.4%), followed by the results from Akin et al. [2012] (15.7%) and Jose et al. [2009] (15.6%). The HL of the 95% CI in most studies falls to the left of the IVL, the HL of the research crosses the IVL, and a small number of studies have the HL of the 95% CI to the right of the IVL. In the 13 included studies, group A included 510 patients, group B included 526 patients, and any complications found within the 2 groups were statistically heterogeneous (Chi2=14.82; I2=60%; P=0.02). In Figure 9, the CES is on the left side of the IVL, with an OR value of 0.46 and a 95% CI of 0.22–0.96. The REM analysis shows lower rates of complication for patients in group B than for patients in group A (Z=2.06; P=0.04).
Figure 10 shows a funnel chart which compares the complications of the patients in groups A and B. The circles concentrated in the top area suggest the accuracy of the included studies was high. The asymmetrical distribution on both sides of the IVL is reflective of publication bias.
Comparison of hemodynamic indicators
The hemodynamic indices of patients after cardiac surgery compared in Figure 11 are average pulse pressure (APP), average heart rate (AHR), and blood oxygen saturation (BOS). The Mostafa et al. [2013] study accounted for the highest percentage of included patients (18.1%), followed by the studies of Stephanie et al. [2005] (17.1%) and Noorizan et al. [2011] (16.8%). The HL of the 95% CI in most studies is seen to fall to the left of the IVL. There is no crossover between the HL or IVL included in the research, and the HL of the 95% CI is on the right of the IVL in a small number of studies. Among the 13 included studies, there were 300 patients in group A and 317 patients in group B, reflecting statistical heterogeneity in hemodynamic indicators (Chi2=59.50; I2=92%; P<0.00001). The CES is on the left side of the IVL, while the OR value and the 95% CI are –6.10, and –10.32 to –1.88, respectively. REM analysis suggests that the hemodynamic indices of patients in group B were superior to those of group A (Z=2.83; P=0.005).
Figure 12 shows a funnel chart of the hemodynamic indices. The concentration of circles in the top area indicates that the accuracy of the included studies was high. The circles are found on both sides of the midline, but they are generally asymmetrical, which is indicative of publication bias.
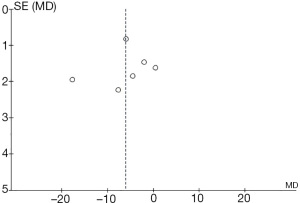
Comparison of anesthesia-induced sedative effects
Figure 13 shows the comparison of sedation levels for patients in group A and group B during the induction of anesthesia. Research from Akin et al. [2012] accounted for the highest percentage of included patients (39.5%), followed by patients in the Ghali et al. [2011] (19.3%) and Koruk et al. [2010] studies (17.4%). Most studies show the 95% CI to the right of the IVL and crossing over the HL, with the HL of the 95% CI of a small number of studies falling to the left of the IVL. There were 200 patients in each group. The sedation of patients during induction of anesthesia was statistically heterogeneous (Chi2=6.45; I2=38%; P=0.17) with the CES seen on the right side of the IVL, and an OR value and 95% CI of 2.12 and 1.36–3.31, respectively. The FEM analysis shows greater sedative effects for patients in group B (Z=3.31; P=0.0009).
Figure 14 shows a funnel chart comparing the sedative effect on patients in the 2 groups during induction of anesthesia. The circles are distributed on both sides and they are roughly symmetrical, showing no publication bias.

Discussion
Research from Ghali et al. [2011] (15) found that midazolam has many limitations and defects as a preoperative drug, potentially causing postoperative restlessness, behavioral changes, cognitive dysfunction, and respiratory depression. At present, there are many sedative drugs available for cardiac surgery patients, with dexmedetomidine, midazolam, and propofol being the more commonly used medications (16). As a highly selective α2 receptor agonist, dexmedetomidine has good sedative and antisympathetic effects. Good sedative treatment can effectively alleviate patient discomfort and reduce the impact of external stimuli (17), and dexmedetomidine is gradually gaining attention, as it has been shown to both maintain hemodynamic stability and has an inability to induce respiratory depression (18).
Of the 13 articles in this study, 12 adopted a randomized controlled grouping method and only 1 adopted a retrospective analysis method, which might have introduced bias to our study. However, the effect on the results was minimal, as using a meta-analysis to synthesize the literature neutralizes the differences among the studies sampled from different populations. A meta-analysis can assign different weights to results, which increases a sample size and improves the credibility of conclusions (19). The number of articles included in this study was limited due to the objective influence of the literature, so sample sizes should be increased for any future investigations.
The Boolean logic search method was applied to conduct a meta-analysis on the 13 articles that described using midazolam and propofol as controls to explore the sedative effect of dexmedetomidine on patients undergoing cardiac surgery. As a result of this meta-analysis, the complications of group A and group B were statistically heterogeneous (Chi2=14.82; I2=60%; P=0.02). Patients in group B showed markedly lower complication rates (Z=2.06; P=0.04), which indicates that dexmedetomidine used for postoperative sedation effectively reduces the incidence of postoperative complications in patients. Heterogeneity of the sedative effects during anesthesia induction for patients in the 2 groups was statistically significant (Chi2=6.45; I2=38%; P=0.17), with the sedative effect of group B observed to be greater than that in group A (Z=3.31; P=0.0009). These results are consistent with the findings of Zhang et al. [2020] (20), which showed that dexmedetomidine has a better sedative effect on patients and that it could also be used to largely reduce both the incidence of MVT and complications in patients after cardiac surgery.
Conclusions
The Boolean logic search method was applied to conduct a meta-analysis on the 13 articles which described using midazolam and propofol as controls, with the chosen articles being used to examine the sedative effect of dexmedetomidine on patients undergoing cardiac surgery. The results revealed that dexmedetomidine can significantly reduce the mechanical ventilation time and the incidence of complications in patients after cardiac surgery, and has a high safety and good sedative effect on patients. However, the meta-analysis was limited, as every article selected included a case-control study, so there was inherent survival bias. Furthermore, patients with varying types of heart disease and predisposing factors were included, which contributed to a reduced CES. Follow-up analysis of prospective cardiac surgery patients should consider the increased sedative effect of dexmedetomidine on patients to improve the analysis. Overall, our findings may provide a theoretical basis and data support for the future clinical treatment of heart disease.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://dx.doi.org/10.21037/apm-21-1850
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apm-21-1850). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Djaiani G, Silverton N, Fedorko L, et al. Dexmedetomidine versus Propofol Sedation Reduces Delirium after Cardiac Surgery: A Randomized Controlled Trial. Anesthesiology 2016;124:362-8. [Crossref] [PubMed]
- Liu X, Xie G, Zhang K, et al. Dexmedetomidine vs propofol sedation reduces delirium in patients after cardiac surgery: A meta-analysis with trial sequential analysis of randomized controlled trials. J Crit Care 2017;38:190-6. [Crossref] [PubMed]
- Nguyen J, Nacpil N. Effectiveness of dexmedetomidine versus propofol on extubation times, length of stay and mortality rates in adult cardiac surgery patients: a systematic review and meta-analysis. JBI Database System Rev Implement Rep 2018;16:1220-39. [Crossref] [PubMed]
- Elgebaly AS, Sabry M. Sedation effects by dexmedetomidine versus propofol in decreasing duration of mechanical ventilation after open heart surgery. Ann Card Anaesth 2018;21:235-42. [Crossref] [PubMed]
- Zhu Z, Zhou H, Ni Y, et al. Can dexmedetomidine reduce atrial fibrillation after cardiac surgery? A systematic review and meta-analysis. Drug Des Devel Ther 2018;12:521-31. [Crossref] [PubMed]
- Brock L. Dexmedetomidine in Adult Patients in Cardiac Surgery Critical Care: An Evidence-Based Review. AACN Adv Crit Care 2019;30:259-68. [Crossref] [PubMed]
- Shehabi Y, Ruettimann U, Adamson H, et al. Dexmedetomidine infusion for more than 24 hours in critically ill patients: sedative and cardiovascular effects. Intensive Care Med 2004;30:2188-96. [Crossref] [PubMed]
- Corbett SM, Rebuck JA, Greene CM, et al. Dexmedetomidine does not improve patient satisfaction when compared with propofol during mechanical ventilation. Crit Care Med 2005;33:940-5. [Crossref] [PubMed]
- Herr DL, Sum-Ping ST, England M. ICU sedation after coronary artery bypass graft surgery: dexmedetomidine-based versus propofol-based sedation regimens. J Cardiothorac Vasc Anesth 2003;17:576-84. [Crossref] [PubMed]
- Shehabi Y, Grant P, Wolfenden H, et al. Prevalence of delirium with dexmedetomidine compared with morphine based therapy after cardiac surgery: a randomized controlled trial (DEXmedetomidine COmpared to Morphine-DEXCOM Study). Anesthesiology 2009;111:1075-84. [Crossref] [PubMed]
- Hu X, Jia M, Zhao Y, et al. Sedative effect of dexmedetomidine and midazolam after cardiac surgery. Ningxia Journal of Medicine 2011;33:967-9.
- Maldonado JR, Wysong A, van der Starre PJ, et al. Dexmedetomidine and the reduction of postoperative delirium after cardiac surgery. Psychosomatics 2009;50:206-17. [Crossref] [PubMed]
- Anger KE, Szumita PM, Baroletti SA, et al. Evaluation of dexmedetomidine versus propofol-based sedation therapy in mechanically ventilated cardiac surgery patients at a tertiary academic medical center. Crit Pathw Cardiol 2010;9:221-6. [Crossref] [PubMed]
- Abd Aziz N, Chue MC, Yong CY, et al. Efficacy and safety of dexmedetomidine versus morphine in post-operative cardiac surgery patients. Int J Clin Pharm 2011;33:150-4. [Crossref] [PubMed]
- Ghali AM, Mahfouz AK, Al-Bahrani M. Preanesthetic medication in children: A comparison of intranasal dexmedetomidine versus oral midazolam. Saudi J Anaesth 2011;5:387-91. [Crossref] [PubMed]
- Akin A, Bayram A, Esmaoglu A, et al. Dexmedetomidine vs midazolam for premedication of pediatric patients undergoing anesthesia. Paediatr Anaesth 2012;22:871-6. [Crossref] [PubMed]
- Mostafa MG, Morsy KM. Premedication with intranasal dexmedetomidine midazolam and ketamine for children undergoing bone marrow biopsy and aspirate. Egyptian J Anaesth 2013;29:131-5. [Crossref]
- Koruk S, Mizrak A, Gul R, et al. Dexmedetomidine-ketamine and midazolam-ketamine combinations for sedation in pediatric patients undergoing extracorporeal shock wave lithotripsy: a randomized prospective study. J Anesth 2010;24:858-63. [Crossref] [PubMed]
- Mountain BW, Smithson L, Cramolini M, et al. Dexmedetomidine as a pediatric anesthetic premedication to reduce anxiety and to deter emergence delirium. AANA J 2011;79:219-24. [PubMed]
- Zhang J, Yu Q, Liu Y, et al. Comparison of ED50 of intranasal dexmedetomidine sedation in children with acyanotic congenital heart disease before and after cardiac surgery. Nan Fang Yi Ke Da Xue Xue Bao 2020;40:864-8. [PubMed]
(English Language Editors: J. Collie and J. Gray)











