
Comparison of magnetic resonance elastography and transient elastography in the diagnosis of hepatic fibrosis: a systematic review and meta-analysis
Introduction
Liver fibrosis occurs when liver cells regenerate after repeated damage and there is an increase in the diffuse deposition and abnormal distribution of extracellular matrix proteins such as collagens, glycoproteins, and proteoglycans in the liver (1,2). Liver fibrosis is a key step in the pathological repair of chronic liver injury and an important link in the development of various chronic liver diseases to cirrhosis. Moreover, it is a factor influencing the prognosis of patients with chronic liver diseases (3-5).
Transient elastography (TE) technology consists of 3 key parts: a transducer that generates ultrasonic waves and acts as an ultrasonic receiver; a probe on the transducer that emits low-frequency vibration waves; and a software program for data recording and analysis (6-8). TE is widely used in the assessment of various organs in the body. Instantaneous elastography technology has the advantages of being non-invasive, painless, fast, and convenient for bedside and outpatient examinations; further, the results have good repeatability. Instantaneous elastography technology can not only be used to non-invasively diagnose liver fibrosis but also to monitor the development of liver disease and evaluate the effect of anti-fibrosis therapy (9-11).
Magnetic resonance elastography (MRE) is a non-invasive and quantitative imaging method for examining soft tissue elasticity and structure. MRE is the longest established and most widely used imaging method in the diagnosis and classification of liver fibrosis (12,13). In the progression of liver fibrosis, liver stiffness increases significantly due to the aggregation of collagen fibers. The elasticity value obtained by MRE can distinguish liver fibrosis (F1–F3) and liver cirrhosis with good sensitivity and specificity. Owing to its non-invasive characteristics compared to traditional liver biopsy, MRE has been used for clinical testing and diagnosis in hepatic fibrosis (14,15).
Since there are few reports comparing MRE and TE in the diagnosis of liver fibrosis, this meta-analysis was conducted to gain a better understanding of the overall diagnostic performance of these 2 techniques in hepatic fibrosis and to help maximize their clinical utility. We present the following article in accordance with the PRISMA reporting checklist (available at https://dx.doi.org/10.21037/apm-21-1176).
Methods
Literature search strategy
Systematic literature searches were carried out to identify studies comparing the diagnostic performance of MRE and ultrasonic TE in hepatic fibrosis that were published between 2007 and 2020. Databases including PubMed, EMBASE, the Cochrane Library, and China National Knowledge were searched using the following keywords: (I) MRE; (II) TE; (III) hepatic fibrosis. All of these words were assembled with the Boolean operator “and”. In order to maximize the specificity and sensitivity of each search, the researcher also checked the reference lists of retrieved studies to identify other potential studies of relevance that were not included in the initial search results.
Study selection criteria
The inclusion criteria for this meta-analysis were: (I) studies comparing MRE with TE for the diagnosis of hepatic fibrosis; (II) studies reporting the diagnostic sensitivity and specificity of the 2 imaging methods; and (III) studies comparing the parameters for each fibrosis stage.
The exclusion criteria were: (I) studies that did not compare the sensitivity and specificity of MRE and TE; (II) study participants had diseases other than liver fibrosis; (III) they are duplicate data; (IV) limited or insufficient research data.
Data extraction and quality assessment
The full texts of the manuscripts (16-23) were read independently by 2 reviewers, and any discrepancies were resolved through discussion with another author. The following data were extracted from each eligible study: first author’s name, country of origin, publication year, sample size, study time, and age and sex of the study participants.
Statistical analysis
Review Manager 5.2 was employed to estimate the effects of selected articles. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for continuous results. Heterogeneity across studies was assessed using the Q statistic and the I2 statistic, which is a quantitative measure of inconsistency among studies. Studies with an I2 statistic of >75% were considered to possess a high degree of heterogeneity; studies with an I2 statistic of 50–75% were considered to possess a moderate degree of heterogeneity; and studies with an I2 statistic of 25–50% were considered to possess a low degree of heterogeneity. If I2>50%, potential sources of heterogeneity were identified through sensitivity analyses conducted by omitting 1 study at a time and investigating the effect on the overall pooled estimate. If heterogeneity was not significant, the Mantel-Haenszel fixed-effects model was applied to compare ORs and 95% CIs; otherwise, a random-effects model was used.
Results
Search process
A total of 914 articles were retrieved in the electronic database search. After careful reading of their titles and abstracts, 72 articles were considered as being potentially relevant. After reviewing the articles against the eligibility criteria, we excluded 64 articles due to the article type, or having an ineligible research design or insufficient data. Finally, 8 eligible articles were included in this meta-analysis. The flow chart in Figure 1 details the process of identifying eligible studies as well as the reasons for the inclusion and exclusion of studies.
Characteristics of the included studies
Table 1 summarizes the characteristics of the included studies. The 8 studies included in the meta-analysis involved a total of 847 participants (432 men and 415 women), with the study sample sizes ranging between 62 and 207 (16-23).
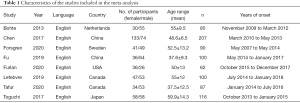
Full table
Results of quality assessment
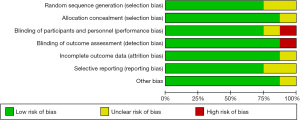
The Cochrane risk of bias assessment tool was used to assess the included studies (Figures 2 and 3). Overall bias was not found in any article. In view of the bias assessment, no selection bias, performance bias, or reporting bias was found, and only 1 study showed detection bias and 1 study showed attrition bias. None of the included studies showed a high risk of bias (8).
Results of heterogeneity testing
Meta-analysis of the diagnostic sensitivity of MRE and TE in stage F0–F1 liver fibrosis
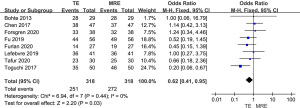
As shown in Figure 4, all 8 studies included diagnostic sensitivity of MRE and TE in stage F0–F1 liver fibrosis. The results showed that the sensitivity of MRE was higher than that of TE for the diagnosis of stage F0–F1 liver fibrosis (OR =0.62, 95% CI: 0.41–0.95, P=0.03; I2=0%).
Meta-analysis of the diagnostic sensitivity of MRE and TE in stage F2–F4 liver fibrosis
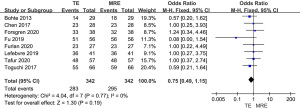
As shown in Figure 5, all 8 studies included diagnostic sensitivity of MRE and TE in stage F2–F4 liver fibrosis. No difference in sensitivity was observed between MRE and TE in the diagnosis of stage F2–F4 liver fibrosis (OR =0.75, 95% CI: 0.49–1.15, P=0.19; I2=0%).
Meta-analysis of the diagnostic specificity of MRE and TE in stage F0–F1 liver fibrosis
As shown in Figure 6, all 8 studies included diagnostic specificity of MRE and TE in stage F0–F1 liver fibrosis. No difference was found in specificity between MRE and TE in the diagnosis of stage F0–F1 liver fibrosis (OR =0.93, 95% CI: 0.64–1.35, P=0.70; I2=0%).
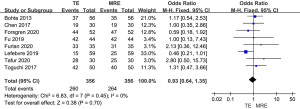
Meta-analysis of the diagnostic specificity of MRE and TE in stage F2–F4 liver fibrosis
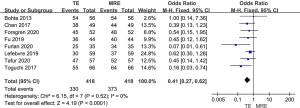
As shown in Figure 7, all 8 studies included diagnostic specificity of MRE and TE in stage F2–F4 liver fibrosis. The results showed that the specificity of MRE was higher than that of TE for the diagnosis of stage F2–F4 liver fibrosis (OR =0.41, 95% CI: 0.27–0.62, P<0.0001; I2=0%).
Results of sensitivity and publication bias analyses
The results of the meta-analysis showed that MRE had higher sensitivity than TE for the diagnosis of hepatic fibrosis. As shown in the Figure 8 the heterogeneity may be attributed to the differences in the results of the studies. After the exclusion of Tafur’s 2020 study, I2 changed from 0% to 14%, and the P value changed from 0.03 to 0.04 (Figure 8), indicating the reliability of the results of this article.
A funnel plot was drawn to assess publication bias in the 8 studies included in this meta-analysis (Figure 9). The good symmetry of the funnel chart showed that there was no publication bias in the included studies (Figure 9).
Discussion
The results of this meta-analysis showed that the diagnostic sensitivity of MRE was higher than that of TE for stage F0–F1 liver fibrosis. Furthermore, in the diagnosis of stage F2–F4 liver fibrosis, MRE also showed higher specificity than TE. Our results are consistent with those of previous reports (24-26).
Pathologically, liver fibrosis refers to the excessive proliferation and abnormal deposition of extracellular matrix components in liver tissues, which causes pathological structural changes and/or functional abnormalities in the liver. In essence, it is a repair response of the liver to chronic damage. The symptoms of liver fibrosis are closely related to the primary disease and the condition of the liver at the time (27,28). Some patients may experience symptoms such as fatigue, loss of appetite, and discomfort in the right upper abdomen, whereas patients with mild liver fibrosis may not exhibit any symptoms.
During the MRE examination process, a slight mechanical vibration (30–70 Hz) is transmitted to the tissue to be studied through an external vibration device, and the dynamic propagation of vibration waves in the tissue is collected by the MRI machine (29-31). In post-processing, the structure and elasticity of the tissue can be reconstructed based on the appearance (wavelength and amplitude) of the vibration wave in the tissue; through this, the softness or hardness of the tissue can be quantified. In the diagnosis of liver fibrosis, MRE also has the advantages of having simple and easy operation, strong reproducibility, and few human-dependent factors, as well as high accuracy. Moreover, it can obtain the elasticity of both the whole liver and different regions of the liver. The quantitative index is more comprehensive than liver biopsy or ultrasound elastography, as it is not affected by factors like obesity and ascites (32-34).
Instantaneous elastography is a type of ultrasound elastography technology that can be used to determine the staging of liver fibrosis through the detection of liver tissue stiffness. It has the advantages of being non-traumatic and rapid. In the assessment of the degree of liver fibrosis, the transmission speed of the shear wave in the liver is directly related to the stiffness of the liver tissue (35,36): the greater the stiffness of the liver tissue, the faster the propagation speed of the shear wave and the greater the elasticity value. Antiviral therapy can improve liver fibrosis, and the degree of liver fibrosis is an important factor in assessing the prognosis of patients (37,38). Therefore, it is of great significance to assess liver fibrosis before and during antiviral therapy.
In conclusion, the results of our meta-analysis show that MRE is superior to TE for the diagnosis of liver fibrosis of different stages in terms of sensitivity and specificity. However, there are some limitations to this meta-analysis. Firstly, it did not take into account comparisons of different age groups, and secondly, the details of heterogeneity were not analyzed. Future studies will seek to address these limitations.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://dx.doi.org/10.21037/apm-21-1176
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apm-21-1176). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ichikawa S, Motosugi U, Morisaka H, et al. Validity and Reliability of Magnetic Resonance Elastography for Staging Hepatic Fibrosis in Patients with Chronic Hepatitis B. Magn Reson Med Sci 2015;14:211-21. [Crossref] [PubMed]
- Myers RP, Elkashab M, Ma M, et al. Transient elastography for the noninvasive assessment of liver fibrosis: a multicentre Canadian study. Can J Gastroenterol 2010;24:661-70. [Crossref] [PubMed]
- Pavlov CS, Casazza G, Nikolova D, et al. Transient elastography for diagnosis of stages of hepatic fibrosis and cirrhosis in people with alcoholic liver disease. Cochrane Database Syst Rev 2015;1:CD010542 [Crossref] [PubMed]
- Wong VW, Chan HL. Transient elastography. J Gastroenterol Hepatol 2010;25:1726-31. [Crossref] [PubMed]
- Cescon M, Colecchia A, Cucchetti A, et al. Value of transient elastography measured with FibroScan in predicting the outcome of hepatic resection for hepatocellular carcinoma. Ann Surg 2012;256:706-12; discussion 712-3. [Crossref] [PubMed]
- Elkrief L, Rautou PE, Ronot M, et al. Prospective comparison of spleen and liver stiffness by using shear-wave and transient elastography for detection of portal hypertension in cirrhosis. Radiology 2015;275:589-98. [Crossref] [PubMed]
- Schon HT, Bartneck M, Borkham-Kamphorst E, et al. Pharmacological Intervention in Hepatic Stellate Cell Activation and Hepatic Fibrosis. Front Pharmacol 2016;7:33. [Crossref] [PubMed]
- Baik SK, Lim YL, Cho YZ, et al. Effect of mesenchymal stem cell on hepatic fibrosis in thioacetamide-induced cirrhotic rat model. J Hepatol 2015;62:S335. [Crossref]
- Murao S, Mitsufuji K, Hirata K, et al. Numerical Analysis by Coupling a Particle Method with the FEM for Design of MRE Soft Actuator. Transactions Institute of Electrical Engineers of Japan 2017;50:52-61.
- Doycheva I, Cui J, Nguyen P, et al. Non-invasive screening of diabetics in primary care for NAFLD and advanced fibrosis by MRI and MRE. Aliment Pharmacol Ther 2016;43:83-95. [Crossref] [PubMed]
- Morse CG, McLaughlin M, Matthews L, et al. Nonalcoholic Steatohepatitis and Hepatic Fibrosis in HIV-1-Monoinfected Adults With Elevated Aminotransferase Levels on Antiretroviral Therapy. Clin Infect Dis 2015;60:1569-78. [Crossref] [PubMed]
- Schwabl P, Bota S, Salzl P, et al. New reliability criteria for transient elastography increase the number of accurate measurements for screening of cirrhosis and portal hypertension. Liver Int 2015;35:381-90. [Crossref] [PubMed]
- Ichikawa S, Motosugi U, Morisaka H, et al. MRI-based staging of hepatic fibrosis: Comparison of intravoxel incoherent motion diffusion-weighted imaging with magnetic resonance elastography. J Magn Reson Imaging 2015;42:204-10. [Crossref] [PubMed]
- Dulai PS, Sirlin CB, Loomba R. MRI and MRE for non-invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: Clinical trials to clinical practice. J Hepatol 2016;65:1006-16. [Crossref] [PubMed]
- Pepin KM, Ehman RL, McGee KP. Magnetic resonance elastography (MRE) in cancer: Technique, analysis, and applications. Prog Nucl Magn Reson Spectrosc 2015;90-91:32-48. [Crossref] [PubMed]
- Tafur M, Cheung A, Menezes RJ, et al. Risk stratification in primary sclerosing cholangitis: comparison of biliary stricture severity on MRCP versus liver stiffness by MR elastography and vibration-controlled transient elastography. Eur Radiol 2020;30:3735-47. [Crossref] [PubMed]
- Lefebvre T, Wartelle-Bladou C, Wong P, et al. Prospective comparison of transient, point shear wave, and magnetic resonance elastography for staging liver fibrosis. Eur Radiol 2019;29:6477-88. [Crossref] [PubMed]
- Bohte AE, de Niet A, Jansen L, et al. Non-invasive evaluation of liver fibrosis: a comparison of ultrasound-based transient elastography and MR elastography in patients with viral hepatitis B and C. Eur Radiol 2014;24:638-48. [Crossref] [PubMed]
- Fu F, Li X, Chen C, et al. Non-invasive assessment of hepatic fibrosis: comparison of MR elastography to transient elastography and intravoxel incoherent motion diffusion-weighted MRI. Abdom Radiol (NY) 2020;45:73-82. [Crossref] [PubMed]
- Toguchi M, Tsurusaki M, Yada N, et al. Magnetic resonance elastography in the assessment of hepatic fibrosis: a study comparing transient elastography and histological data in the same patients. Abdom Radiol (NY) 2017;42:1659-66. [Crossref] [PubMed]
- Furlan A, Minervini MI, Rachakonda V, et al. Liver stiffness measurement in patients with nodular regenerative hyperplasia undergoing magnetic resonance elastography. Abdominal Radiology 2020;55:81-92.
- Chen J, Yin M, Talwalkar JA, et al. Diagnostic Performance of MR Elastography and Vibration-controlled Transient Elastography in the Detection of Hepatic Fibrosis in Patients with Severe to Morbid Obesity. Radiology 2017;283:418-28. [Crossref] [PubMed]
- Forsgren MF, Nasr P, Karlsson M, et al. Biomarkers of liver fibrosis: prospective comparison of multimodal magnetic resonance, serum algorithms and transient elastography. Scand J Gastroenterol 2020;55:848-59. [Crossref] [PubMed]
- Stockdale AJ, Phillips RO, Beloukas A, et al. Liver Fibrosis by Transient Elastography and Virologic Outcomes After Introduction of Tenofovir in Lamivudine-Experienced Adults With HIV and Hepatitis B Virus Coinfection in Ghana. Clin Infect Dis 2015;61:883-91. [Crossref] [PubMed]
- Kennedy P, Barnhill E, Gray C, et al. Magnetic resonance elastography (MRE) shows significant reduction of thigh muscle stiffness in healthy older adults. Geroscience 2020;42:311-21. [Crossref] [PubMed]
- Trautwein C, Friedman SL, Schuppan D, et al. Hepatic fibrosis: Concept to treatment. J Hepatol 2015;62:S15-24. [Crossref] [PubMed]
- Adinolfi LE, Giordano MG, Andreana A, et al. Hepatic fibrosis plays a central role in the pathogenesis of thrombocytopenia in patients with chronic viral hepatitis. Br J Haematol 2001;113:590-5. [Crossref] [PubMed]
- Kawada N. Evolution of hepatic fibrosis research. Hepatol Res 2011;41:199-208. [Crossref] [PubMed]
- Lafrance-Vanasse J, Williams GJ, Tainer JA. Envisioning the dynamics and flexibility of Mre11-Rad50-Nbs1 complex to decipher its roles in DNA replication and repair. Prog Biophys Mol Biol 2015;117:182-93. [Crossref] [PubMed]
- Yang J, Sun S, Tian T, et al. Development of a novel multi-layer MRE isolator for suppression of building vibrations under seismic events. Mechanical Systems & Signal Processing 2016;s70–71:811-20.
- Ichikawa S, Motosugi U, Morisaka H, et al. Comparison of the diagnostic accuracies of magnetic resonance elastography and transient elastography for hepatic fibrosis. Magn Reson Imaging 2015;33:26-30. [Crossref] [PubMed]
- Li D, Zhang B, Xing X, et al. Combining MeDIP-seq and MRE-seq to investigate genome-wide CpG methylation. Methods 2015;72:29-40. [Crossref] [PubMed]
- Liu F, Wang X, Wu G, et al. Coffee Consumption Decreases Risks for Hepatic Fibrosis and Cirrhosis: A Meta-Analysis. PLoS One 2015;10:e0142457 [Crossref] [PubMed]
- Khalaf N, White D, Kanwal F, et al. Coffee and Caffeine Are Associated With Decreased Risk of Advanced Hepatic Fibrosis Among Patients With Hepatitis C. Clin Gastroenterol Hepatol 2015;13:1521-31.e3. [Crossref] [PubMed]
- Matsuura K, De Giorgi V, Schechterly C, et al. Circulating let-7 levels in plasma and extracellular vesicles correlate with hepatic fibrosis progression in chronic hepatitis C. Hepatology 2016;64:732-45. [Crossref] [PubMed]
- Saxena NK, Anania FA. Adipocytokines and hepatic fibrosis. Trends Endocrinol Metab 2015;26:153-61. [Crossref] [PubMed]
- Liu H, Fu J, Hong R, et al. Acoustic Radiation Force Impulse Elastography for the Non-Invasive Evaluation of Hepatic Fibrosis in Non-Alcoholic Fatty Liver Disease Patients: A Systematic Review & Meta-Analysis. PLoS One 2015;10:e0127782 [Crossref] [PubMed]
- Xing Z, Yu M, Sun S, et al. A hybrid magnetorheological elastomer-fluid (MRE-F) isolation mount: development and experimental validation. Smart Materials & Structures 2016;25:15026. [Crossref]
(English Language Editor: J. Reynolds)






