
This article has an erratum available at: http://dx.doi.org/10.21037/apm-2021-08 the article has been update on 2021-11-06 at here.
Clinical effect and safety of venous access ports and peripherally inserted central catheters in patients receiving tumor chemotherapy: a systematic review and meta-analysis
Introduction
Chemotherapy is a necessary cancer treatment, but the chemotherapy cycle is long, and chemotherapy drugs cause intense irritation to blood vessels (1,2). Extravasation causes injection site pain, swelling, erythema, and in serious cases causes blackening or blistering of the skin and the formation of ulcers. Another drawback of chemotherapy includes the requirement of surgical debridement and skin grafting. Therefore, patients must have access to safer, more reliable, and long-term intravenous infusions (3,4).
Peripherally inserted central catheters (PICC) and venous access port (PORT) are 2 suitable catheterization methods for intravenous chemotherapy, long-term repeated blood transfusion, and parenteral nutrition (5,6). PICCs run from the peripheral arm to the central vein, which effectively protects the vein of the upper limb and reduces the number of punctures, inflammation, and pain (7). The clinical effect of PICC has shown improved outcomes compared to a traditional disposable infusion set by positively impacting the quality of life and nursing care of patients who receive the long-term infusion (8). However, when the PICC is implanted, catheter rupture, prolapse, or lumen obstruction may occur, which increases the probability of infection (9). In these scenarios, early extubation is required, which affects the continuity of treatment and reduces the quality of life. A PICC catheter can be placed in the body for 3–12 months under normal conditions, a relatively short time for patients who need long-term deep venous catheterization (10).
PORTs can be wholly implanted into the body, providing long-term stable venous access and a closed intravenous infusion system (11). PORTs can be classified as arm PORTs or chest PORTs, depending on the location of the port. At present, the chest PORT is the most common (12). PORTs and PICCs are made of high-grade silica gel, which has a low rejection rate and high biocompatibility with the human body (13). There is no significant difference in the success rate of PORT and PICC catheterization.
We found several published studies concerning PICCs and PORTs in cancer patients receiving chemotherapy. However, there were minimal meta-analyses on the clinical effects and safety of PORTs and PICCs in patients receiving tumor chemotherapy. Therefore, we conducted this meta-analysis. This research is different from other similar article since it compare PICCs and PORTs in several aspects and update the included articles that published in recent years. We present the following article in accordance with the PRISMA reporting checklist (available at https://dx.doi.org/10.21037/apm-21-1926).
Methods
Search strategy
An academic librarian developed search strategies using PubMed, EMBASE, Cochrane Library, and China National Knowledge Infrastructure from the inception of this study to 31 May 2021. The keywords included: (venous access port OR PORT) AND chemotherapy AND (cancer OR tumor) AND (peripherally inserted central catheter OR PICC). A comprehensive search was performed for literature, with no limitation on the year of publication or language. To achieve maximum sensitivity of the search strategy and find all relevant studies, we also performed a manual screen of all reference lists to identify potentially related studies further.
Selection process
Double-blind, randomized trials of PICCs and PORTs in adult cancer patients (≥18 years) receiving chemotherapy were eligible for inclusion in this study if they had undergone a clinical effects evaluation, with complication as a primary or secondary outcome. The selection criteria included: (I) adult population with cancer; (II) study involving a survey, questionnaire, interview, or focus group; (III) measured and reported patient clinical effects and safety; and (IV) patients receiving chemotherapy. Patients with different types of catheters were excluded from this study. Studies that were published as abstracts only were included after contacting the authors for more detailed information. If multiple publications from the same cohort were available, we extracted data from the largest or most recent data set.
Data collection and quality assessment
The two authors of this study independently reviewed the contents of the officially published versions of all included studies. These studies also used data extraction tables based on the Cochrane Consumer and Communication Review Group data extraction templates to filter specific inclusion criteria. The two authors resolved their differences through discussion; if we cannot reach an agreement, the third author is invited to decide. Use standardized tables to extract information about design, settings, study population (such as recruitment period, age, gender), the number of participants, follow-up period, and results.
The risk of bias of the included randomized controlled trials (RCT) was assessed using an improved version of the Cochrane Collaboration risk of bias tool. Two co-authors independently evaluated the risk of bias for all included randomized controlled trials. If there is a disagreement, recheck the original text and then discuss it to reach a consensus.
Statistical analysis
We use Review Manager version 5.2 software (Cochrane Collaboration, 2011) for statistical analysis. To measure the consistency of effect size [OR (odds ratio) and SMD (standard mean deviation)], a paired meta-analysis was performed using the DerSimonian and Laird random-effects model to calculate the direct comparison between placebo and drug category or individual treatment. The OR and SMD summary estimate of 95% confidence interval (CI). To assess the heterogeneity, we calculated the Cochrane statistic and the I2 statistic. I2 values of 25%, 50%, and 75% are considered low, medium, and high heterogeneity. If heterogeneity is observed, the random-effects model (P<0.1) is used, and if there is no inter-study heterogeneity, the fixed effects model is used. Publication bias was represented graphically by funnel plots of the standard difference in means versus the standard error. Visual inspection of funnel plot asymmetry was performed to address the possible small-study effect. Lastly, we performed a sensitivity analysis by omitting studies conducted by Patel et al.’s study in 2014 to examine the robustness of the disparities, respectively.
Results
Study selection
Of 651 articles, 45 were eligible for full-text screening, with 10 original studies ultimately meeting the eligibility criteria of randomized controlled trials (Figure 1). Next, entire papers were selected to be closely reviewed and matched with the eligibility criteria for future data extraction. Finally, 10 eligible studies were used in this meta-analysis (14-23).
Study characteristics
Two authors independently reviewed the papers that were included in the final analyses and extracted relevant data from the papers. The extracted information included the first author’s name, patient’s age and gender, country of origin, year of publication, sample size, study duration, and primary outcome.
A total of 2,585 patients were procured for the meta-analysis, including 945 patients treated with PICC technology and 1,640 patients treated with PORT technology (Table 1).
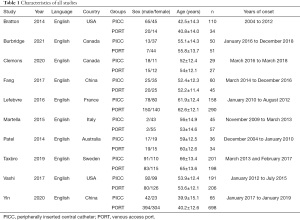
Full table
Results of quality assessment
The Cochrane risk of bias assessment tool was used to evaluate risk in the included studies. Scores ranged from 7 to 8, and 9 studies scored more than 7 points. Figures 2 and 3 present a summary of the risk of bias for each included study. Overall, the results showed that the included articles were of good quality.
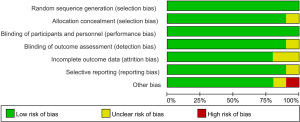
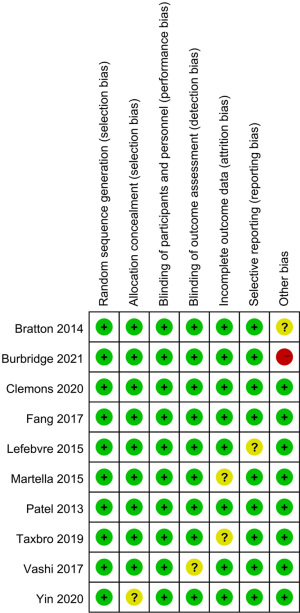
Results of heterogeneity test
Heterogeneity analysis of procedure time between PICC and PORT
To analyze the difference in procedure time between the PICC and PORT groups, we performed a meta-analysis to calculate the mean difference (MD) using the fixed-effect model. The overall MD was −5.55 with 95% CI, −6.96, −4.14. The P value of the overall effect was <0.00001, I2=0%, which demonstrated that the difference in procedure time between the PICC and PORT groups was significant, and the procedure time in the PORT group was more than that in the PICC group (Figure 4).

Heterogeneity analysis of quality of life (QOL) between PICC and PORT
Similarly, a meta-analysis for the difference in patient satisfaction between the PICC and PORT was conducted. The result showed that there was no significant difference in QOL between the PICC and PORT groups (MD =−1.12, 95% CI, −6.14, 3.91, P=0.66, fixed effect model), and the included studies demonstrated low homogeneity (P=0.23, I2=32%) (Figure 5).
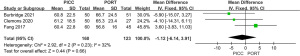
Heterogeneity analysis of occlusion between PICC and PORT
To examine occlusion, 4 studies involving 1,369 patients were analyzed. Meta-analysis showed that there was difference in occlusion between the PICC and PORT groups (MD =5.42, 95% CI, 2.13, 13.75, P=0.0004, random effect model), with insignificant heterogeneity (I2=40%) (Figure 6). Occlusion in PICC group was more than that in PORT group.
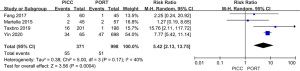
Meta-analysis of thrombosis in the devices
As shown in Figure 7, 7 studies were included. The result showed that thrombosis in the PICC device was greater than that which was observed in the PORT group [risk ratio (RR) =4.37, 95% CI, 2.10, 9.07, P<0.0001, I2=22%, Figure 7].
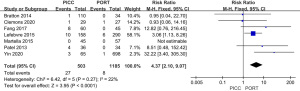
Results of sensitivity analysis and publication bias
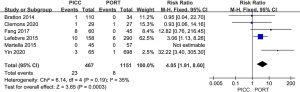
A total of 7 studies reported thrombosis in the device. The forest plot showed that the PICC group exhibited greater thrombosis than the PORT group (RR =4.37, 95% CI, 2.10, 9.07, P<0.0001, I2=22%, Figure 7). We performed a sensitivity analysis by removing Patel et al.’s study in 2014, which had little effect, changing the I2 result from 22% to 35% (Figure 8), which indicated that the results of included articles were robust. We also constructed a funnel plot to evaluate publication bias for thrombosis, and the resultant figure showed a symmetric shape. The P value of the Egger test was 0.348, which indicated no significant publication bias existed in this meta-analysis (Figure 9).
Discussion
Ten studies met the inclusion criteria for evaluating the effects and complications of the use of PICCs and PORTs in cancer patients receiving chemotherapy. Meta-analysis of these studies showed that PORTs required a longer procedure time than PICCs, and PICCs exhibited greater occlusion and thrombosis than PORTs, which indicated that the use of PORTs was safer than PICCs. In contrast, PICCs were more convenient than PORTs. There was no difference in QOL between the PICC and PORT groups, demonstrating that both treatments had similar clinical effects.
PORTs had no special requirements for their use in the selected peripheral vascular conditions. PORT implantation was non-invasive and exhibited a low infection rate, so patients' daily lives were not greatly impacted (24). PICCs require flushing once per week, while PORTs only need to be washed once per month. However, the attending doctor must perform the PORT washing in an operating room, presenting challenges (25). Additionally, PORT procedures must be performed using a dedicated non-destructive needle which increases the economic burden on patients. After the catheter is inserted into the body, it is impossible to observe its status, and the removal process requires surgery (26). In contrast, PICCs can be managed by professionally trained nurses.
In terms of nursing, PICC patients require the attendance of a nurse once per week. This requirement can present challenges if local hospitals do not have the correct conditions for the changing of dressings. In this case, patients would be required to seek out a larger hospital, increasing patients’ travel time and their economic burden (27). Although patients who receive PORTs only need hospital care once every 4 weeks, if they increase their hospital attendance, and receive care every 7 days, PORTs can last up to several years (28). In contrast, the life of PICCs is 3–12 months, causing an increased economic burden on patients (29).
For some patients, the PICC line is the safest infusion therapy. For others, PICC can avoid the risk of invasive surgery required for long-term central catheter placement. Many patients appreciate the benefits of PICC infusion at home and quickly resume normal activities PICCs can avoid pain and injury caused by repeated punctures with short peripheral catheters (29). Cost is also a consideration (30). The advantages of PORT are as follows. Accessing a port is an access port mechanism, not direct access to a vein, which can avoid stab wounds and direct damage to the veins. Ports are obvious and easy to feel, and there will be safer and more effective visits than intravenous sites (31).
Compared with PICCs, PORTs have the following advantages: high quality material, convenient tube, and aesthetically favorable. However, PORTs present disadvantages in their cost and the technology required to implant and maintain the devices. In particular, severe complications can arise during PORT implantation, such as pneumothorax, bleeding, and clipping syndrome (30,31), which are beyond the scope of this study.
The limitations of this study were identified. The cost and complications involved in the operation were not discussed, and these issues need to be addressed. In addition, we did not analyze further details surrounding postoperative complications and long-term survival rates. In summary, PORTs are a safer treatment than PICCs for cancer patients receiving chemotherapy, but there is no significant difference in clinical efficacy between PICCs and PORTs. Due to limitations in the number and quality of included research papers, the conclusion of this study must be confirmed by the use of a larger sample size and a multi-center follow-up controlled trial.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist Available at https://dx.doi.org/10.21037/apm-21-1926
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apm-21-1926). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Prager D, Hertzberg RW. Spontaneous intravenous catheter fracture and embolization from an implanted venous access port and analysis by scanning electron microscopy. Cancer 1987;60:270-3. [Crossref] [PubMed]
- Zhou J, Qian S, He W, et al. Implanting totally implantable venous access port via the internal jugular vein guided by ultrasonography is feasible and safe in patients with breast cancer. World J Surg Oncol 2014;12:378. [Crossref] [PubMed]
- Chang YF, Lo AC, Tsai CH, et al. Higher complication risk of totally implantable venous access port systems in patients with advanced cancer - a single institution retrospective analysis. Palliat Med 2013;27:185-91. [Crossref] [PubMed]
- Chopra V, Anand S, Krein SL, et al. Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med 2012;125:733-41. [Crossref] [PubMed]
- Jiang Z, Chen FH. Research Progress of Expression of XIAP in Non-small Cell Lung Cancer and Chemotherapy Drug Resistance. Medical Recapitulate 2012;29:189-85.
- Yue HE, Sun YP, Li N, et al. Comparison of the effects of PICC and venous access port in patients with hematological malignancies. Chinese Journal of Nursing 2012;47:1001-3.
- Narducci F, Jean-Laurent M, Boulanger L, et al. Totally implantable venous access port systems and risk factors for complications: a one-year prospective study in a cancer centre. Eur J Surg Oncol 2011;37:913-8. [Crossref] [PubMed]
- Goltz JP, Scholl A, Ritter CO, et al. Peripherally placed totally implantable venous-access port systems of the forearm: clinical experience in 763 consecutive patients. Cardiovasc Intervent Radiol 2010;33:1159-67. [Crossref] [PubMed]
- Nocito A, Wildi S, Rufibach K, et al. Randomized clinical trial comparing venous cutdown with the Seldinger technique for placement of implantable venous access ports. Br J Surg 2009;96:1129-34. [Crossref] [PubMed]
- Evans RS, Sharp JH, Linford LH, et al. Risk of Symptomatic DVT Associated With Peripherally Inserted Central Catheters. Chest 2010;138:803-10. [Crossref] [PubMed]
- Hoekstra H, Lemmers N, Gels M, et al. 1210 Complications of venous access port (VAP) in patients with non-seminomatous testicular germ cell tumours. Eur J Cancer 1995;31:S253. [Crossref]
- Douard MC, Arlet G, Longuet P, et al. Diagnosis of venous access port-related infections. Clin Infect Dis 1999;29:1197-202. [Crossref] [PubMed]
- Racadio JM, Doellman DA, Johnson ND, et al. Pediatric peripherally inserted central catheters: complication rates related to catheter tip location. Pediatrics 2001;107:E28 [Crossref] [PubMed]
- Bratton J, Johnstone PA, McMullen KP. Outpatient management of vascular access devices in children receiving radiotherapy: complications and morbidity. Pediatr Blood Cancer 2014;61:499-501. [Crossref] [PubMed]
- Burbridge B, Lim H, Dwernychuk L, et al. Comparison of the Quality of Life of Patients with Breast or Colon Cancer with an Arm Vein Port (TIVAD) Versus a Peripherally Inserted Central Catheter (PICC). Curr Oncol 2021;28:1495-506. [Crossref] [PubMed]
- Clemons M, Stober C, Kehoe A, et al. A randomized trial comparing vascular access strategies for patients receiving chemotherapy with trastuzumab for early-stage breast cancer. Support Care Cancer 2020;28:4891-9. [Crossref] [PubMed]
- Fang S, Yang J, Song L, et al. Comparison of three types of central venous catheters in patients with malignant tumor receiving chemotherapy. Patient Prefer Adherence 2017;11:1197-204. [Crossref] [PubMed]
- Lefebvre L, Noyon E, Georgescu D, et al. Port catheter versus peripherally inserted central catheter for postoperative chemotherapy in early breast cancer: a retrospective analysis of 448 patients. Support Care Cancer 2016;24:1397-403. [Crossref] [PubMed]
- Martella F, Salutari V, Marchetti C, et al. A retrospective analysis of trabectedin infusion by peripherally inserted central venous catheters: a multicentric Italian experience. Anticancer Drugs 2015;26:990-4. [Crossref] [PubMed]
- Patel GS, Jain K, Kumar R, et al. Comparison of peripherally inserted central venous catheters (PICC) versus subcutaneously implanted port-chamber catheters by complication and cost for patients receiving chemotherapy for non-haematological malignancies. Support Care Cancer 2014;22:121-8. [Crossref] [PubMed]
- Taxbro K, Hammarskjöld F, Thelin B, et al. Clinical impact of peripherally inserted central catheters vs implanted port catheters in patients with cancer: an open-label, randomised, two-centre trial. Br J Anaesth 2019;122:734-41. [Crossref] [PubMed]
- Vashi PG, Virginkar N, Popiel B, et al. Incidence of and factors associated with catheter-related bloodstream infection in patients with advanced solid tumors on home parenteral nutrition managed using a standardized catheter care protocol. BMC Infect Dis 2017;17:372. [Crossref] [PubMed]
- Yin L, Li J. Central Venous Catheter Insertion in Colorectal Cancer Patients, PICC or PC? Cancer Manag Res 2020;12:5813-8. [Crossref] [PubMed]
- Ramos S. Cancer chemoprevention and chemotherapy: dietary polyphenols and signalling pathways. Mol Nutr Food Res 2008;52:507-26. [Crossref] [PubMed]
- Schweickert WD, Herlitz J, Pohlman AS, et al. A randomized, controlled trial evaluating postinsertion neck ultrasound in peripherally inserted central catheter procedures. Crit Care Med 2009;37:1217-21. [Crossref] [PubMed]
- Dubois J, Rypens F, Garel L, David M, Lacroix J, Gauvin F. Incidence of deep vein thrombosis related to peripherally inserted central catheters in children and adolescents. CMAJ 2007;177:1185-90. [Crossref] [PubMed]
- Kreis H, Loehberg CR, Lux MP, et al. Patients' attitudes to totally implantable venous access port systems for gynecological or breast malignancies. Eur J Surg Oncol 2007;33:39-43. [Crossref] [PubMed]
- Windich-Biermeier A, Sjoberg I, Dale JC, et al. Effects of distraction on pain, fear, and distress during venous port access and venipuncture in children and adolescents with cancer. J Pediatr Oncol Nurs 2007;24:8-19. [Crossref] [PubMed]
- Inaba Y, Yamaura H, Sato Y, et al. Central venous access port-related complications in outpatient chemotherapy for colorectal cancer. Jpn J Clin Oncol 2007;37:951-4. [Crossref] [PubMed]
- Chow LM, Friedman JN, Macarthur C, et al. Peripherally inserted central catheter (PICC) fracture and embolization in the pediatric population. J Pediatr 2003;142:141-4. [Crossref] [PubMed]
- Chemaly RF, de Parres JB, Rehm SJ, et al. Venous thrombosis associated with peripherally inserted central catheters: a retrospective analysis of the Cleveland Clinic experience. Clin Infect Dis 2002;34:1179-83. [Crossref] [PubMed]
(English Language Editors: B. Maizey and J. Chapnick)




