Cut points for mild, moderate, and severe pain among cancer and non-cancer patients: a literature review
Introduction
Pain is a common and debilitating complaint that remains a diagnostic and therapeutic challenge to clinicians. Pain is associated with an extensive differential diagnosis that includes cancer and non-cancer etiologies such as osteoarthritis, lower back pain, and diabetic neuropathy (1-4). Studies have shown that 50-90% of patients with advanced and metastatic cancer require pain management (5-7). Regardless of etiology, pain is a common and often distressing symptom that can significantly impact a patient’s quality of life.
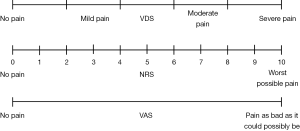
The visual analogue scale (VAS), verbal descriptor scale (VDS), and numeric rating scale (NRS) are three frequently-used tools for assessing pain severity. As shown in Figure 1, the VAS consists of a 10 cm line anchored by terms representing the extremes of pain severity—“no pain” and “pain as bad as it could possibly be” on which patients record their pain intensity. The VDS allows patients to verbally describe their pain by choosing from a list of adjectives corresponding to different categories of pain severity: no pain, mild pain, moderate pain or severe pain. The NRS is an 11-point numerical scale ranging from 0 to 10, where 0 indicates no pain and 10 indicates the worst possible pain.
All three pain-rating scales have demonstrated their validity, reliability, and applicability in research and clinical practice (8,9). However, the NRS has been shown to be particularly reliable especially among patients with low health literacy (10). The NRS is also simple to score, and can be administered both orally and in written form (10). In comparison, the VAS may be difficult to administer to frail patients or those with limited vision. Additionally, the VDS are a relatively crude measure that usually lists only several adjectives for the patient to describe their pain. Despite these limitations, many clinicians prefer to use the mild, moderate, and severe categorization in the VDS because of its resemblance to our daily communication style. Moreover, many treatment recommendations for patients with pain are based on this simple threefold classification (mild, moderate and severe pain). For example, the VDS is utilized in the World Health Organization’s analgesic ladder (11,12) and cancer guidelines by the Agency for Health Care Policy and Research (13). For this reason, we have chosen to evaluate this threefold VDS in this study instead of other formats which contain more descriptive adjectives.
Establishing clear cut points (CPs) for mild, moderate, and severe pain on the NRS is important in research and clinical practice. Firstly, CPs can be used as a guide in selection and initiation of pain treatment. Secondly, they can be used to quantify changes in a patient’s functional status and quality of life (14). Additionally, changes in pain levels between mild, moderate, and severe pain intensity categories may be a more clinically meaningful measure of treatment effectiveness compared to the absolute change on the pain severity scale (9).
Currently, there is a lack of consensus regarding the optimal CPs for mild, moderate, and severe pain on the NRS pain scale. Serlin et al. (10) were the first to analyze the NRS pain intensity ratings with respect to the interference of pain with daily activities among patients with cancer. The authors used CPs of 4 and 6 to define mild-moderate and moderate-severe pain respectively. They also showed that the relationship between pain intensity and functional interference brought on by pain was non-linear. Hence, changes in pain intensity of the same interval on different parts of the NRS scale may not necessarily result in a similar change in functional interference. Furthermore, they suggested that, cancer pain did not begin to significantly result in disruption of patients’ lives until it approached a specific CP of 5. CPs has also been determined for various chronic pain conditions, such as diabetic neuropathy, phantom limb pain, osteoarthritis, and low-back pain (3,4,9,15,16). Although these studies were carried out using similar methods introduced by Serlin et al. (10), CPs appeared to be highly dependent on the affected anatomic sites and underlying illnesses.
We conducted a literature review to (I) summarize all studies that identified CP values for mild, moderate, and severe pain intensity on the NRS, and (II) recommend optimal CPs for mild, moderate, and severe pain separately among cancer and non-cancer patient populations.
Methods
Search strategy
Ovid MEDLINE and OLDMEDLINE (inception to May 2015, week 1) and Ovid EMBASE and EMBASE classic (inception to 2015 week 18) were searched for all literature pertaining to pain scale CPs among patients with and without cancer. The search consisted of Medical Subject Headings and keywords for pain scales and pain measurement, CPs, and pain grading or severity.
Selection criteria
All identified studies were screened by title and abstract and full-text articles were then reviewed for eligibility. Studies were included if they reported NRS CPs for patients with cancer or non-cancer conditions leading to acute or chronic pain. Any article utilizing statistical analysis to determine the optimal CP pair for their target patient group was included. Non-English studies and non-original studies (i.e., reviews) were excluded. To conduct a comprehensive literature search, we searched the bibliographies of included articles for relevant cross-references.
We defined CPs as the upper bound of a pain intensity category. For example, CPs of 5 and 7 (CP5, 7) represent ranges of 1-5, 6-7, and 8-10 for mild, moderate, and severe pain categories, respectively.
Data extraction and identification of CPs
Data on patient demographics and pain characteristics (i.e., type of pain, measure of pain, pain CPs) were extracted from the included articles. The primary outcome was the CP used to define categories of pain intensity on the NRS. A secondary outcome was the functional interference caused by pain, which was assessed in the literature using the Brief Pain Inventory (BPI). The BPI incorporated the 11-point NRS, where patients rate their current, worst, least, and average pain intensity. In addition, the level of functional interference caused by pain (e.g., general activity, mood, walking ability, sleep, enjoyment of life, normal work, and relations with others) was also rated by patients. In the included studies, all possible combinations of pain intensity CPs were then created and tested in relations to the set of seven interference items from the BPI using different statistical analytical approaches.
Results
We identified 1,556 articles through the original search, of which 21 met the inclusion criteria. Reasons for exclusions were: duplicate papers, studies providing insufficient information, studies on other pain assessment tools, and non-original studies. The additional search produced six supplementary articles. After applying the aforementioned criteria, 27 relevant articles were selected for inclusion. Out of these, seven publications studied patients with cancer and twenty publications studied patients with non-cancer conditions.
Among the included studies, 15 studies (3,4,9,10,15-25) used multivariate analysis of variance (MANOVA), 7 studies (2,26-31) used analysis of variance (ANOVA), and the remaining 5 studies (32-36) utilized other statistical approaches such as generalized linear model (GLM). CPs were identified by multivariate analysis among pain-severity categories yielding the largest F-ratio for the between-category effect on total pain-related interference score as indicated by Pillai’s trace, Wilks’ Lambda, and Hostelling’s trace F statistics.
Seven studies (10,17-19,25,32,33) investigated the CPs between mild-moderate and moderate-severe pain in patients with cancer. Among these seven studies, four of them (10,18,19,32) examined patients with metastatic cancer and three of them (18,19,32) involved patients with bone metastasis. The remaining three studies examined patients with generalized cancer pain (17,25,33). Table 1 summarizes CPs used in these studies.
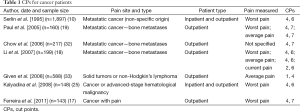
Full table
Seven studies (10,17-19,25,32,33) reported ten CPs, with two studies (18,19) reporting more than one CP. For mild-moderate pain, the CP ranged from 1 to 4. The average CP was 3.5±1.08. The most frequently recommended CP was 4 (8 of 10 results, 80%). For moderate-severe pain, the CP ranged from 4 to 7. The average CP was 6.2±0.92. The most frequently used CPs was 6 (5 of 10 results, 50%).
Table 2 summarizes CPs used in studies of patients with pain caused by non-cancer conditions. Twenty studies (2-4,9,15,16,20-24,26-31,34-36) reported 34 CPs with 12 reporting more than one CP. For mild-moderate pain, the CP ranged from 2 to 5. The average CP was 3.62±0.78. The most frequently used CP was 4 (18 of 34 results, 52.9%) and 3 (10 of 34 results, 29.4%). For moderate-severe pain, the CP ranged from 4 to 8. The average CP was 6.5±0.99. The most frequently presented CP was 6 (14 of 34 results, 41.2%) and 7 (10 of 34 results, 29.4%).

Full table
Discussion
Cancer-related pain
For cancer-related mild-moderate pain, CP4 appears to be the optimal CP (10,17-19,25,32). For moderate-severe pain, CP6 or CP7 was the most frequently used CP (10,17-19,25,32). Among the six studies, only Given et al. (33) reported a significantly different CP (CP1, 4). The discrepancy between results may reflect the variations in cancer diagnoses of the target patient population. Given et al. reported on patients with solid tumors including non-Hodgkin’s lymphoma whereas the target population of most of the other studies were patients with bone metastases (18,19,32). Additionally, differences in the type or aggressiveness of treatment may also affect the reported CPs. Different analytical approaches may also yield different CPs. Given et al. used a GLM while the other cancer-related studies typically used MANOVA to generate the optimal CPs.
Zelman et al. (4) suggested that ‘worst pain’ should be used for CP derivation instead of ‘average pain’ and ‘current pain’ for cancer-related pain. This is because in patients with cancer, especially with advanced metastatic disease, ‘worst pain’ often exacerbates acutely as breakthrough pain. These events are often indicators of the severity and progression of the disease. The level of ‘worst pain’ can also signify the effectiveness of analgesic medications. Subgroup analysis of only studies that investigated CPs for ‘worst pain’ (10,17-19,25) yielded similar results, CP4 for mild-moderate, and CP6 or CP7 for moderate-severe pain. However, breakthrough pain and acute exacerbation can be confounding factors in complicating the overall cancer-related pain experienced by the patient. The use of worst pain CPs for cancer-related pain should be investigated in future studies.
Non-cancer pain
For non-cancer pain, average pain levels may better reflect patients’ daily experience with functional and emotional interference. Zelman et al. (4) proposed that ‘average pain’ should be used to derive CPs. Among the 20 included studies, 14 (2-4,9,15,16,20-22,26,28-30,34) reported a total of 19 average pain CPs of the average pain scale, with some studies reporting multiple CPs. Subgroup analysis showed that for mild-moderate pain, the CP ranged from 2 to 5, and CP3 and CP4 contributed to the majority (15 of 19 results, 78.9%) of CPs. The moderate-severe CP ranged from 4 to 8, and CP6 and CP7 contributed to the majority of CPs (14 of 19 results, 73.7%). Again, the use of average pain CPs for non-cancer pain should be evaluated further.
Numerous studies (19,26) reported a variety of CPs depending on the disease, pathophysiology, and mechanism of pain. Neuropathic pain occurs when there is actual damage or compression of the nerves or the central nervous system. This contrasts with nociceptive pain which is caused by the activation of pain receptors when there is damage of non-neural tissues (37). Among the 20 studies on non-cancer patients, there are only seven studies that included neuropathic pain on an average pain scale including phantom limb pain, diabetic peripheral neuropathy, spinal cord injury, multiple sclerosis and trigeminal neuralgia. Subgroup analysis of these seven studies (3,9,15,26,28-30) yielded a narrow range of CPs. The mild-moderate CP ranged from 2 to 4, with CP3 contributing to 4 of 7 studies (57.1%) (3,15,29,30). Moderate-severe CPs ranged from 5 to 8, with CP6 being the most commonly reported CP (3 of 7 studies, 42.9%) (3,15,29). There were eight studies (2,4,9,16,21,22,28,34) that reported CPs for nociceptive pain on an average pain scale including patients suffering from back and neck pain, osteoarthritis of knee and hip, non-dental orofacial pain and postoperative pain. Among these eight studies, the mild-moderate CP ranged from 2 to 5, with CP4 contributing to 7 of 10 CPs (70%), and the moderate-severe CPs ranged from 4 to 8, with CP6 contributing to 4 of 10 CPs (40%).
Numerous factors could have contributed to the variability in the reported CPs. Firstly, patient populations varied in terms of inpatients or outpatients. Secondly, using a different pain scale (average and worst pain) will also produce different CPs. As mentioned above, some studies have suggested that ‘average pain is more appropriate for measuring chronic pain, while ‘worst pain’ is a better measure for unstable pain. Furthermore, different CPs could reflect different etiologies of pain (neuropathic versus nociceptive pain). Even with the same pathophysiology (e.g., osteoarthritis), various pain sites (e.g., knee and hip) may also influence the CPs. Finally for the same anatomic pain site, differences in disease stage/progression or treatment (e.g., joint replacement surgery and non-steroidal anti-inflammatory drugs) could yield different CPs (4,16).
Since the CPs chosen were tested in relation with the interference items from the BPI, the extent of functional or emotional interference caused by a certain kind of pain could also impact the resultant CPs. For example, trigeminal neuralgia is often described as one of the most painful and distressing conditions (38). Pain and discomfort in the facial region can cause significant interference with daily function and quality of life by affecting speech, eating, sleeping, and other important function. Hence, the CPs for trigeminal neuralgia would be expected to be lower than other conditions that are associated with less functional interference. The extent of interference also depends on personal pain tolerance and situational necessity. Finally, differences in study methodology and statistical testing could affect the generation of CPs. Most studies only tested combinations of CPs which were believed to be the most reasonable in order to save time; this may have limited the possibility of other outcomes.
To our knowledge, this is the first comprehensive review of studies examining pain severity CPs for the NRS for both patients with cancer and non-cancer conditions. Together with the already existing guidelines and research, the resulting CPs are all based on optimal group boundaries and may not be the best CPs to represent individual patients. Future investigations would benefit from larger and more diverse patient populations to explore the different CPs on the pain intensity scale for patients with cancer and non-cancer pain. Additional studies are required to determine whether the establishment of CPs for pain severity should be based on the specific components of pain problem being investigated (persistent stable pain versus persistent pain with breakthrough crisis) or the etiology of the pain problem (neuropathic versus nociceptive versus pain with mixed etiology). Future studies should also analyze both average and worst pain scores to determine the optimal CPs specific to each disease on that certain pain scale. Longitudinal studies should also be carried out to evaluate the stability of these CPs over time to determine if they can be used practically in clinical practice. In addition, a wider range of CP combinations should be tested and analyzed using standardized statistical tools to generate more meaningful comparisons across studies.
Conclusions
Establishing optimal CPs for varying levels for pain intensity is important for assisting clinicians in the development and evaluation of treatment options. A wide range of CPs for mild, moderate, and severe pain categories were identified in the literature among both cancer and non-cancer patients populations. However, the majority of cancer studies recommended CP4 for mild-moderate and pain and either CP6 or CP7 for moderate-severe pain, while most non-cancer studies recommended CP4 and CP6 as optimal CPs for mild-moderate and moderate-severe pain. These CPs are to be selected and used with caution in daily clinical practice based on different patient groups and pain etiologies. Further studies are needed to delineate more accurate and precise CPs for pain intensity among specific patient populations.
Acknowledgements
We thank the generous support of the Bratty Family Fund, Michael and Karyn Goldstein Cancer Research Fund, Joey and Mary Furfari Cancer Research Fund, Pulenzas Cancer Research Fund, Joseph and Silvana Melara Cancer Research Fund, and Ofelia Cancer Research Fund.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Oldenmenger WH, de Raaf PJ, de Klerk C, et al. Cut points on 0-10 numeric rating scales for symptoms included in the Edmonton Symptom Assessment Scale in cancer patients: a systematic review. J Pain Symptom Manage 2013;45:1083-93. [PubMed]
- Turner JA, Franklin G, Heagerty PJ, et al. The association between pain and disability. Pain 2004;112:307-14. [PubMed]
- Zelman DC, Dukes E, Brandenburg N, et al. Identification of cut-points for mild, moderate and severe pain due to diabetic peripheral neuropathy. Pain 2005;115:29-36. [PubMed]
- Zelman DC, Hoffman DL, Seifeldin R, et al. Development of a metric for a day of manageable pain control: derivation of pain severity cut-points for low back pain and osteoarthritis. Pain 2003;106:35-42. [PubMed]
- Bradley N, Davis L, Chow E. Symptom distress in patients attending an outpatient palliative radiotherapy clinic. J Pain Symptom Manage 2005;30:123-31. [PubMed]
- Edrington JM, Paul S, Dodd M, et al. No evidence for sex differences in the severity and treatment of cancer pain. J Pain Symptom Manage 2004;28:225-32. [PubMed]
- Wells N. Pain intensity and pain interference in hospitalized patients with cancer. Oncol Nurs Forum 2000;27:985-91. [PubMed]
- McDowell I. Measuring Health: A Guide to Rating Scales and Questionnaires. 3 edition. New York: Oxford University Press, 2006.
- Jensen MP, Smith DG, Ehde DM, et al. Pain site and the effects of amputation pain: further clarification of the meaning of mild, moderate, and severe pain. Pain 2001;91:317-22. [PubMed]
- Serlin RC, Mendoza TR, Nakamura Y, et al. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain 1995;61:277-84. [PubMed]
- World Health Organization. Cancer pain relief and palliative care: Report of the WHO expert committee on cancer pain relief and active supportive care (Technical Report Series 804). Geneva, Switzerland, 1990. Available online: http://apps.who.int/iris/bitstream/10665/39524/1/WHO_TRS_804.pdf
- World Health Organization. Cancer pain relief: with a guide to opioid availability. Geneva, Switzerland, 1996.
- Jacox A, Carr DB, Payne R. New clinical-practice guidelines for the management of pain in patients with cancer. N Engl J Med 1994;330:651-5. [PubMed]
- Anderson KO. Role of cutpoints: why grade pain intensity? Pain 2005;113:5-6. [PubMed]
- Hoffman DL, Sadosky A, Dukes EM, et al. How do changes in pain severity levels correspond to changes in health status and function in patients with painful diabetic peripheral neuropathy? Pain 2010;149:194-201. [PubMed]
- Kapstad H, Hanestad BR, Langeland N, et al. Cutpoints for mild, moderate and severe pain in patients with osteoarthritis of the hip or knee ready for joint replacement surgery. BMC Musculoskelet Disord 2008;9:55. [PubMed]
- Ferreira KA, Teixeira MJ, Mendonza TR, et al. Validation of brief pain inventory to Brazilian patients with pain. Support Care Cancer 2011;19:505-11. [PubMed]
- Li KK, Harris K, Hadi S, et al. What should be the optimal cut points for mild, moderate, and severe pain? J Palliat Med 2007;10:1338-46. [PubMed]
- Paul SM, Zelman DC, Smith M, et al. Categorizing the severity of cancer pain: further exploration of the establishment of cutpoints. Pain 2005;113:37-44. [PubMed]
- Brown KE, Swift I, Spark MJ. Pain Severity Cut-Points and Analgesic Use by Community-Dwelling People for Chronic Pain. J Pharm Pract Res 2012;42:196-9.
- Dihle A, Helseth S, Paul SM, et al. The exploration of the establishment of cutpoints to categorize the severity of acute postoperative pain. Clin J Pain 2006;22:617-24. [PubMed]
- Fejer R, Jordan A, Hartvigsen J. Categorising the severity of neck pain: establishment of cut-points for use in clinical and epidemiological research. Pain 2005;119:176-82. [PubMed]
- Mendoza TR, Chen C, Brugger A, et al. Lessons learned from a multiple-dose post-operative analgesic trial. Pain 2004;109:103-9. [PubMed]
- Zalon ML. Mild, moderate, and severe pain in patients recovering from major abdominal surgery. Pain Manag Nurs 2014;15:e1-12. [PubMed]
- Kalyadina SA, Ionova TI, Ivanova MO, et al. Russian Brief Pain Inventory: validation and application in cancer pain. J Pain Symptom Manage 2008;35:95-102. [PubMed]
- Alschuler KN, Jensen MP, Ehde DM. Defining mild, moderate, and severe pain in persons with multiple sclerosis. Pain Med 2012;13:1358-65. [PubMed]
- Alschuler KN, Otis JD. An examination of the impact of clinically significant levels of posttraumatic stress disorder symptomatology on the classification of pain as mild, moderate, or severe in a sample of veterans with chronic pain. Psychol Serv 2014;11:273-80. [PubMed]
- Brailo V, Zakrzewska JM. Grading the intensity of nondental orofacial pain: identification of cutoff points for mild, moderate, and severe pain. J Pain Res 2015;8:95-104. [PubMed]
- Forchheimer MB, Richards JS, Chiodo AE, et al. Cut point determination in the measurement of pain and its relationship to psychosocial and functional measures after traumatic spinal cord injury: a retrospective model spinal cord injury system analysis. Arch Phys Med Rehabil 2011;92:419-24. [PubMed]
- Hanley MA, Masedo A, Jensen MP, et al. Pain interference in persons with spinal cord injury: classification of mild, moderate, and severe pain. J Pain 2006;7:129-33. [PubMed]
- Hirschfeld G, Zernikow B. Variability of "optimal" cut points for mild, moderate, and severe pain: neglected problems when comparing groups. Pain 2013;154:154-9. [PubMed]
- Chow E, Doyle M, Li K, et al. Mild, moderate, or severe pain categorized by patients with cancer with bone metastases. J Palliat Med 2006;9:850-4. [PubMed]
- Given B, Given CW, Sikorskii A, et al. Establishing mild, moderate, and severe scores for cancer-related symptoms: how consistent and clinically meaningful are interference-based severity cut-points? J Pain Symptom Manage 2008;35:126-35. [PubMed]
- Gerbershagen HJ, Rothaug J, Kalkman CJ, et al. Determination of moderate-to-severe postoperative pain on the numeric rating scale: a cut-off point analysis applying four different methods. Br J Anaesth 2011;107:619-26. [PubMed]
- Jones KR, Vojir CP, Hutt E, et al. Determining mild, moderate, and severe pain equivalency across pain-intensity tools in nursing home residents. J Rehabil Res Dev 2007;44:305-14. [PubMed]
- Palos GR, Mendoza TR, Mobley GM, et al. Asking the community about cutpoints used to describe mild, moderate, and severe pain. J Pain 2006;7:49-56. [PubMed]
- Millan MJ. The induction of pain: an integrative review. Prog Neurobiol 1999;57:1-164. [PubMed]
- Eller JL, Raslan AM, Burchiel KJ. Trigeminal neuralgia: definition and classification. Neurosurg Focus 2005;18:E3. [PubMed]