
Diagnostic value of transvaginal three-dimensional ultrasound combined with color Doppler ultrasound for early cesarean scar pregnancy
Introduction
Cesarean scar pregnancy (CSP) is a rare form of ectopic pregnancy, which is one of the long-term complications of cesarean section. It is generally accepted that fertilized eggs and embryos implanted in the scar caused by a prior cesarean section will be surrounded by myometrium and fibrous tissue, thus resulting in CSP (1-4). This is a disease without specific clinical symptoms in the early stage of pregnancy. With the progression of pregnancy, the villi continue to adhere and implant into the myometrium, which contributes to the increased risk of uterine rupture, massive hemorrhage, and even life-threatening situation (5). Therefore, early diagnosis and effective treatment are vital for the management of this disease.
Vial et al. (6) concluded that there are two types of CSP, endogenic and exogenic (6). The endogenic type involves progression of gestational tissue to the scar, uterine cavity, or uterine isthmus but with shallow depth of implantation into the myometrium. The exogenic type involves attachment of the gestational sac to the area around the scar, and trophoblast cells invade deeply into the lower uterine segment or even into the bladder. However, Xiang (7) asserted that it is more appropriate to classify CSP into three types, including the above two types, and another subtype of CSP with mixed echogenic mass. The last subtype is often misdiagnosed as trophoblastic tumor and is mainly characterized by mixed echogenic mass in the lower uterine segment. In this study, in order to diagnose CSP more accurately, we divided CSP into the following three types: CSP with partial implantation of the gestational sac, CSP with complete implantation of the gestational sac, and CSP with mixed echogenic mass.
With the development of science and technology and the increased attention of obstetricians and gynecologists to CSP, its early diagnosis rate is increasing (8). Imaging examination is currently the main way to diagnose CSP, including two-dimensional ultrasound (2D-US), three-dimensional ultrasound (3D-US), contrast-enhanced ultrasound (CEUS), and magnetic resonance imaging (MRI) (9). Among them, 3D-US has become an important examination method for the diagnosis of CSP due to its simplicity, convenience, lack of radiation, high reproducibility, and good visualization. According to statistics, by comparison with transabdominal ultrasound (US), the probe of transvaginal US has a high frequency and can be in close contact with pelvic organs. Transvaginal 3D-US with continuous, multiplanar, and multi-angle observation of CSP can make up for the lack of transvaginal 2D-US imaging in diagnosis of early CSP, thus obtaining a clearer image. This technique can clearly show the patient’s uterine adnexa to assess the condition of pregnancy, giving it a high diagnostic value (10-12). With the development and maturation of the related technologies in recent years, transvaginal 3D-US has been increasingly introduced to the diagnosis of early CSP (13,14). However, by comparison with transvaginal 3D-US, transvaginal 3D color Doppler US can show the source, shape, and distribution density of blood flow in gestational tissue. The latter can more comprehensively visualize the situation of CSP to improve the diagnostic accuracy of early CSP, so it has critical reference value for clinical condition evaluation and treatment options.
Therefore, we selected patients with suspected CSP admitted to our hospital as the study participants to compare the diagnostic effects of transvaginal 3D-US alone and transvaginal 3D-US combined with color Doppler US. Through this comparison, the diagnostic value of transvaginal 3D-US combined with color Doppler US for CSP was probed, aiming to improve the diagnostic accuracy as well as clinical evaluation and treatment of CSP. We present the following article in accordance with the STARD reporting checklist (available at https://dx.doi.org/10.21037/apm-21-2208).
Methods
Baseline data
A total of 142 suspected CSP patients initially diagnosed in our hospital from January 2017 to December 2019 were collected as the study participants. All participants were randomly divided into a Control group (n=71) diagnosed by 3D-US and a Combination group (n=71) diagnosed by 3D-US combined with color Doppler US. This study was approved by the Medical Ethics Committee of Maternal and Child Healthcare Hospital Hubei (2021-IEC-LW004). All procedures performed in this study involving human participants were conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was provided by all patients and their families.
Screening criteria
The inclusion criteria were as follows: (I) initially diagnosed as suspected CSP; (II) age >24 years; (III) previous history of cesarean section; (IV) pregnancy week ≤12, positive results of beta-human chorionic gonadotropin (β-HCG) in blood or urine; (V) with absence of menstruation, abdominal pain, vaginal bleeding; (VI) patients with normal mental health status who were willing to cooperate with this trial.
The exclusion criteria were as follows: (I) recent history of antibiotic or immunosuppressant use; (II) dysfunction or severe infection of heart, lung, liver, or kidneys; (III) malignant tumors; (IV) comorbidities of uterine fibroids, uterine adenomyosis, ovarian lesions; (V) previous history of major gynecological surgery; (VI) patients who could not cooperate with this trial; (VII) incomplete clinical data.
Transvaginal ultrasound
We used GE Voluson E8 real-time three-dimensional color Doppler ultrasound machine and LOGIC E9 ultrasound machine (GE Healthcare, Chicago, IL, USA) with an endocavity probe at a frequency of 5–9 MHz. After emptying their bladder, the participant assumed the lithotomy position. The probe was covered with a condom and slowly inserted into their vagina. Subsequently, the uterus, adnexa, and pelvic cavity were scanned. The following conditions were observed: distance between the gestational sac and the uterine incision, echo of myometrium around the incision, size and shape of the gestational sac, presence or absence of yolk sac or embryo, fetal heartbeat, blood flow and its resistance around the gestational sac, and the thickness of the scar. After that, 3D ultrasonic scanning mode was turned on, and the probe was moved to the center position of the region of interest. The scanning angle and sampling volume were adjusted to locate this region and then tomography imaging was performed. The X, Y, and Z axes were adjusted to obtain satisfactory images. These images were used for diagnostic analysis.
Evaluation of CSP
The assessment of CSP uterine scar was performed by two experienced sonographers, and any differences in the opinions were resolved by discussion to reach consensus. The diagnostic criteria were as follows: (I) no gestational sac in the uterine cavity and cervical canal; (II) gestational sac located at the cesarean scar in the lower uterine segment or located in the anterior wall of the uterine isthmus; (III) scar fissure, and interrupted or thinned myometrium of the anterior wall of the uterus in the lower uterine segment between the gestational sac and the bladder; (IV) positive results of the “organ sliding sign”, that is, when the gestational sac was gently pressed with an US probe, the sac could slide into the cervical canal; (V) the Doppler US showed the annular blood flow signal around the gestational sac, and the pulsed Doppler showed the pulsatility index <1, and the peak velocity >20 cm/s.
Statistical analysis
Using the software SPSS 21.0 (IBM Corp., Armonk, NY, USA), the chi-square test was carried out with outcomes of enumeration data expressed as cases (%). Receiver operating characteristic (ROC) curves were plotted to calculate the area under the curves (AUC). A significant difference was suggested if P<0.05.
Results
Baseline characteristics
A total of 142 patients with suspected CSP were included in the study (Control: n=71; Combination: n=71). No marked differences were found in age, number of cesarean sections, time since the last cesarean section, date of last menstrual period, and content of serum β-HCG between the two groups (Table 1).
Table 1
| Characteristics | Control | Combination | T/z | P value |
|---|---|---|---|---|
| Age (years) | 27.85±2.9357 | 28.61±2.345 | 1.704 | 0.0905 |
| Number of cesarean sections | 1.13±0.34 | 1.15±0.36 | 0.3403 | 0.7341 |
| Time since the last cesarean section (year) | 4.915±1.873 | 5.014±1.478 | 0.3496 | 0.7271 |
| Duration of amenorrhea (day) | 49.94±7.991 | 48.18±5.574 | 1.522 | 0.1302 |
| Serum β-HCG | 20,486.62±2,964.306 | 21,174.08±2,843.362 | 1.41 | 0.1607 |
Comparison of transvaginal 3D-US and 3D-US combined with color Doppler in the diagnosis of CSP
A total of 45 non-CSP patients and 97 CSP patients were confirmed by surgical and pathological examinations. Among them, there were 9 cases of CSP with mixed echogenic mass, 62 cases of CSP with partial implantation of gestational sac, and 26 cases of CSP with complete implantation of gestational sac type. By comparison with the above examination results, 35 were true positive, 13 were false positive, 16 were true negative, and 7 were false negative in the Control group, while 45 cases were true positive, 4 cases were false positive, 21 cases were true negative, and 1 case was false negative in the Combination group (Table 2). It could be seen that 3D-US combined with color Doppler significantly increased the number of true positive and negative cases while decreasing the number of false positive and negative cases. According to further comparison of the diagnostic effect on CSP, the Combination group had higher accuracy, sensitivity, and specificity, which meant a higher diagnostic coincidence rate and a lower misdiagnosis rate (Table 3).
Table 2
| Examination method | Control | Combination | |||
|---|---|---|---|---|---|
| CSP | Non-CSP | CSP | Non-CSP | ||
| Pathological examination | |||||
| CSP | 35 | 13 | 45 | 4 | |
| Non-CSP | 7 | 16 | 1 | 21 | |
| Total | 42 | 29 | 46 | 25 | |
CSP, cesarean scar pregnancy; 3D-US, three-dimensional ultrasound.
Table 3
| Examination method | Accuracy | Sensitivity | Specificity | Missed diagnosis rate | Misdiagnosis rate |
|---|---|---|---|---|---|
| Control | 71.83 (51/71) | 72.92 (35/48) | 69.57 (16/23) | 27.08 (13/48) | 30.43 (7/23) |
| Combination | 92.96 (66/71) | 91.84 (45/49) | 95.45 (21/22) | 8.16 (4/49) | 4.55 (1/22) |
| χ2 | 3.135 | 6.005 | 5.156 | 6.005 | 5.156 |
| P value | 0.0017 | 0.0143 | 0.0232 | 0.0143 | 0.0232 |
3D-US, three-dimensional ultrasound.
Comparison of transvaginal 3D-US and 3D-US combined with color Doppler in the diagnosis of different types of CSP
The diagnostic effects of the two examination methods were further compared in different types of CSP (Table 4). For CSP with mixed echogenic mass, the Control group confirmed 1 case (33.3%), misdiagnosed 2 cases, and found 0 suspected case, while the Combination group confirmed 4 cases (80%), misdiagnosed 1 case, and found 0 suspected case. For CSP with partial implantation of gestational sac, the Control group confirmed 20 cases (74.1%), misdiagnosed 5 cases, and found 2 suspected cases, while the Combination confirmed 26 cases (89.7%), misdiagnosed 1 case, and found 2 suspected cases. For CSP with complete implantation of gestational sac, the Control group confirmed 7 cases (58.3%), misdiagnosed 3 cases, and found 2 suspected cases, while the Combination confirmed 12 cases (100%), misdiagnosed 0 cases, and found 0 suspected case.
Table 4
| Control group | Combination group | ||||||
|---|---|---|---|---|---|---|---|
| Suspected | Misdiagnosed | Confirmed, n (%) | Suspected | Misdiagnosis | Confirmed, n (%) | ||
| CSP with mixed echogenic mass | 0 | 2 | 1 (33.3) | 0 | 1 | 4 (80.0) | |
| CSP with partial implantation of gestational sac | 2 | 5 | 20 (74.1) | 2 | 1 | 26 (89.7) | |
| CSP with complete implantation of gestational sac | 2 | 3 | 7 (58.3) | 0 | 0 | 12 (100.0) | |
CSP, cesarean scar pregnancy; 3D-US, three-dimensional ultrasound.
Collectively, by comparison with the Control group, the Combination group had higher accuracy and diagnostic coincidence rate and lower misdiagnosis rate in the diagnosis of different CSP subtypes.
Comparison of the diagnostic value of transvaginal 3D-US and 3D-US combined with color Doppler in CSP
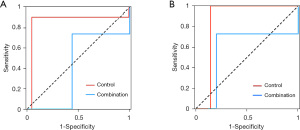
In the diagnosis of CSP, the AUC of the Combination group was larger than that of the Control group (Figure 1A), suggesting that 3D-US combined with color Doppler was more valuable for diagnosing CSP. In the diagnosis of different types of CSP, the AUC of the Combination group was also larger than that of the Control group (Figure 1B), indicating that 3D-US combined with color Doppler was more valuable when diagnostically distinguishing different types of CSP.
Typical cases
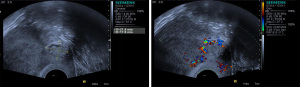
CSP with mixed echogenic mass
The uterus was of normal size or slightly enlarged, with thickened endometrium. The US examination revealed gestational sac-like echo in the uterine cavity and cervical canal. Heterogeneous mass was observed in the lower uterine segment, with nonuniform internal echo and the coexistence of solid and liquid. The mass protruded to the serosal layer, and the thickness of the attachment of the mass to the myometrium of the anterior wall became thinner (all <5 mm) (Figure 2).
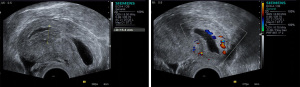
CSP with partial implantation of gestational sac
The uterus was of normal size or slightly enlarged. Most of the gestational sac was located in the uterine cavity, while a small part was located in the lower uterine segment deep to the scar. See Figure 3.
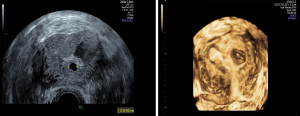
CSP with complete implantation of gestational sac
The uterus was of normal size or slightly enlarged. The US examination revealed no gestational sac-like echo in the uterine cavity and endocervical canal. The gestational sac-like echo was observed in the scar on the anterior wall of lower uterine segment, which was round or oval in shape, and yolk sac, embryo, and fetal heartbeat were validated (Figure 4).
Discussion
In 1978, Larsen first reported and defined CSP (15). In recent years, with the implementation of the 2-child policy and the increase of cesarean section, the incidence of CSP has been increasing yearly in China. In 2003, Jurkovic proposed that among all pregnant women, its incidence was approximately 5.6 per 10,000 (16). In 2011, the Peking Union Medical College Hospital reported that its incidence had elevated to 1 in 1,368 (17), which was higher than in previous reports. In 2014, Jurkovic stated that the incidence of CSP was 0.5/1,000, and estimated that there was 1 case of CSP per 400 pregnancies in the United Kingdom (18).
The pathogenesis of CSP has not been clarified. It is currently believed that the cause of CSP is fissure of the myometrium in the scar. During cesarean section or invasive intrauterine operation, the basal layer and myometrium of the uterus are destroyed and their continuity is interrupted, which affects blood supply in the myometrium. Then, during the healing process, an atrophy or fissure is formed between the destroyed myometrium and the endometrium (19-21). When the gestational tissue implants in or around the fissure, trophoblasts gradually invade the basal layer or even myometrium, and then the gestational tissue is surrounded by myometrium and fibrous tissue in the lower uterine segment to form CSP (22). In the study by Zhang et al. (23), pathological examination of the scar on the anterior wall of the lower uterine segment in 23 cases of CSP revealed a reduced connection of smooth muscle cells and fissures and implantation of the villi between the myometrium. This study indicated that the presence of fissures in the scar may be the main cause of CSP. The myometrium and the fissures in the scar are thin and poorly elastic. Therefore, with the continuous growth of the gestational sac implanted in the scar, the placenta is also locally implanted, causing uterine rupture, and even the bladder is penetrated, which induces massive hemorrhage (24). As a rare disease, it is difficult to carry out research on CSP, which has led to a lack of understanding of its pathogenesis (18). Therefore, early diagnosis and appropriate treatment or early termination of pregnancy are essential for CSP patients.
The diagnosis of CSP needs to be combined with the patient’s history, clinical manifestations, and imaging examination (including transabdominal US, transvaginal US, and MRI) (25). In transvaginal US, the US probe can get close to the vaginal fornix and cervix, and can closely observe the uterine adnexa. Transvaginal US imaging is not obscured by the abdominal wall and intestinal gas, and has a higher resolution. It has the advantages of observing the echogenic structure, blood flow, and muscle wall thickness of CSP by comparison with transabdominal US (26-28). Transvaginal 3D-US is based on the X axis of 2D ultrasound to obtain information of the Y and Z axes. Its imaging has good spatial localization, and can reconstruct the shape, size, and location of abnormal masses. It has specific advantages for the diagnosis of CSP, which can provide more detailed information of ectopic pregnancy and then improve the diagnostic effect of CSP. The 3D color flow imaging, including color and power Doppler imaging and vitreous imaging, can show the gestational tissue with good blood perfusion (different from the gestational tissue with lack of blood supply and inevitable abortion). This kind of imaging can further observe the source, shape, and distribution density of the gestational tissue, as well as the relationship between the gestational tissue and the myometrium of the anterior wall of the uterus, thus reflecting the depth of pregnancy implantation (29).
In this study, in order to clarify the value and advantages of transvaginal 3D-US combined with color Doppler in the diagnosis of CSP, we compared its results with those of transvaginal 3D-US in the diagnosis of CSP. In the diagnosis of CSP, the coincidence rate of transvaginal 3D-US was 71.83%, including 1 case of CSP with mixed echogenic mass, 20 cases of partial implantation of the gestational sac, and 7 cases of complete implantation of the gestational sac; while the coincidence rate of transvaginal 3D-US combined with color Doppler was 92.96%, including 4 cases of CSP with mixed echogenic mass, 26 cases of partial implantation of the gestational sac, and 12 cases of complete implantation of the gestational sac. Our findings revealed that the application of transvaginal 3D-US combined with color Doppler improved the early diagnosis of CSP, and its classification. Further analysis revealed that the Combination group had higher sensitivity, specificity, accuracy, diagnostic coincidence rate, and lower misdiagnosis rate in the diagnosis of CSP. Besides, its ROC curve showed that transvaginal 3D-US combined with color Doppler was more valuable in the diagnosis and classification of CSP.
Transvaginal 3D-US can acquire the volume data to quickly and accurately obtain coronal section information of the region of interest (30). It can visualize the relationship between the gestational tissue mass and endometrium, and the uterine incision. In addition, it can also measure the distance between gestational tissue mass and the myometrium of the anterior wall in the lower uterine segment from multiple angles, thus effectively avoiding the missed diagnosis and misdiagnosis by 2D-US, and providing more effective information for clinical treatment options (31,32). Clinical studies have found that the US results of CSP are manifested as a normal or slightly enlarged uterus and thickened endometrium; if the gestational tissue is located at the incision in the lower uterine segment, the imaging is manifested as thinning of the myometrium of the anterior wall in the lower uterine segment, and a gestational sac or mass with abundant blood flow (18,33). To conclude, transvaginal 3D-US combined with color Doppler had a high diagnostic accuracy for the 3 types of CSP, which could comprehensively observe the condition of CSP. This technique can help clinicians to evaluate the condition and choose appropriate treatment strategies, thus reducing the risk of complications.
Conclusions
In the diagnosis of CSP, by comparison with 2D-US alone, 3D-US combined with color Doppler can improve the sensitivity, specificity, accuracy, and diagnostic coincidence rate, while can reducing the misdiagnosis rate. It can more accurately diagnose the CSP classification. In addition, it can more comprehensively and accurately observe the condition of CSP. Therefore, based on its advantages, 3D-US combined with color Doppler assists the clinical evaluation, diagnosis, and treatment of CSP.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://dx.doi.org/10.21037/apm-21-2208
Data Sharing Statement: Available at https://dx.doi.org/10.21037/apm-21-2208
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apm-21-2208). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Medical Ethics Committee of Maternal and Child Healthcare Hospital Hubei (2021-IEC-LW004). All procedures performed in this study involving human participants were conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was provided by all patients and their families.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Xia J, Wang R, Yu J, et al. Clinical study on 85 cases with caesarean scar pregnancy. Qingdao Medical Journal 2016;48:100-2.
- Timor-Tritsch IE, Khatib N, Monteagudo A, et al. Cesarean scar pregnancies: experience of 60 cases. J Ultrasound Med 2015;34:601-10. [Crossref] [PubMed]
- Birch Petersen K, Hoffmann E, Rifbjerg Larsen C, et al. Cesarean scar pregnancy: a systematic review of treatment studies. Fertil Steril 2016;105:958-67. [Crossref] [PubMed]
- Riaz RM, Williams TR, Craig BM, et al. Cesarean scar ectopic pregnancy: imaging features, current treatment options, and clinical outcomes. Abdom Imaging 2015;40:2589-99. [Crossref] [PubMed]
- Lin P, Huang J, Ding H. Progress of Diagnosis and Treatment for Cesarean Scar Pregnancy. China Foreign Medical Treatment 2017;36:190-2.
- Vial Y, Petignat P, Hohlfeld P. Pregnancy in a cesarean scar. Ultrasound Obstet Gynecol 2000;16:592-3. [Crossref] [PubMed]
- Xiang Y. Classification and treatment of cesarean scar pregnancy. Chinese Journal of Clinical Obstetrics and Gynecology 2012;13:401-4.
- Liu J, Xie H. Diagnosis and treatment of cesarean scar pregnancy. Maternal & Child Health Care of China 2017;32:3480-1.
- Liu D, Yang M, Wu Q. Application of ultrasonography in the diagnosis and treatment of cesarean scar pregnancy. Clin Chim Acta 2018;486:291-7. [Crossref] [PubMed]
- Vora PH, Bansal V. Cesarean scar pregnancy: clinicians challenge. Int J Reprod Contracept Obstet 2017;6:2101-3. [Crossref]
- Patel C, Feldman J, Ogedegbe C. Complicated abdominal pregnancy with placenta feeding off sacral plexus and subsequent multiple ectopic pregnancies during a 4-year follow-up: a case report. J Med Case Rep 2016;10:37. [Crossref] [PubMed]
- Li Y, Wang W, Yang T, et al. Incorporating uterine artery embolization in the treatment of cesarean scar pregnancy following diagnostic ultrasonography. Int J Gynaecol Obstet 2016;134:202-7. [Crossref] [PubMed]
- Green RW, Valentin L, Alcazar JL, et al. Endometrial cancer off-line staging using two-dimensional transvaginal ultrasound and three-dimensional volume contrast imaging: Intermethod agreement, interrater reliability and diagnostic accuracy. Gynecol Oncol 2018;150:438-45. [Crossref] [PubMed]
- Shao M, Cai A, Wang X, et al. Three-dimensional tomographic ultrasound imaging in diagnosis of the cesarean scar pregnancy. Chinese Journal of Medical Imaging Technology 2015;31:893-6.
- Larsen JV, Solomon MH. Pregnancy in a uterine scar sacculus--an unusual cause of postabortal haemorrhage. A case report. S Afr Med J 1978;53:142-3. [PubMed]
- Jurkovic D, Hillaby K, Woelfer B, et al. First-trimester diagnosis and management of pregnancies implanted into the lower uterine segment Cesarean section scar. Ultrasound Obstet Gynecol 2003;21:220-7. [Crossref] [PubMed]
- Wang Y. Progress of diagnosis and treatment of caesarean scar pregnancy. China Medical Herald 2011;8:7-9.
- Jurkovic D. Cesarean scar pregnancy and placent accerete. Ultrasound 0bstet Gynecol 2014,43:361-2.
- Younes G, Goldberg Y, Lavie O, et al. Cesarean Scar Pregnancy: A Case Series of Diagnosis, Treatment, and Results. Journal of Diagnostic Medical Sonography 2018;34:875647931879115 [Crossref]
- Timor-Tritsch IE, Monteagudo A, Cali G, et al. Easy sonographic differential diagnosis between intrauterine pregnancy and cesarean delivery scar pregnancy in the early first trimester. Am J Obstet Gynecol 2016;215:225.e1-7. [Crossref] [PubMed]
- Cali G, Forlani F, Timor-Tritsch IE, et al. Natural history of Cesarean scar pregnancy on prenatal ultrasound: the crossover sign. Ultrasound Obstet Gynecol 2017;50:100-4. [Crossref] [PubMed]
- Shi Y, Jiang Y, Zeng Q, et al. Influencing factors associated with the mode of birth among childbearing women in Hunan Province: a cross-sectional study in China. BMC Pregnancy Childbirth 2016;16:108. [Crossref] [PubMed]
- Zhang N, Yang Q. Analysis of Clinical and Pathological Characteristics in Caesarean Scar Pregnancy. Journal of China Medical University 2011;40:458-61.
- Jain S, Suneja A, Malik R, et al. Cesarean scar pregnancy: a diagnostic dilemma and impending catastrophe. Arch Gynecol Obstet 2014;289:221-2. [Crossref] [PubMed]
- Liu K, Li C, Mo X, et al. Progress in diagnosis and treatment of cesarean scar pregnancy. J Reprod Med 2016;25:660-3.
- Chen L, Li X, Li L, et al. Comparison of transvaginal ultrasound and MRI in diagnosis of the first-trimester cesarean scar pregnancy. Journal of Practical Radiology 2016;32:566-9.
- Wang M, Sun Y. Value of transvaginal color Doppler ultrasonography in the diagnosis and treatment of cesarean scar pregnancy. China Modern Doctor 2016;54:97-8, 102, 3.
- El-Sharkawy M, El-Mazny A, Ramadan W, et al. Three-dimensional ultrasonography and power Doppler for discrimination between benign and malignant endometrium in premenopausal women with abnormal uterine bleeding. BMC Womens Health 2016;16:18. [Crossref] [PubMed]
- Lv H, Huang C, Chen J, et al. Diagnosis and clinical application value of transvaginal three-dimensional color Doppler ultrasound on caesarean scar pregnancy. China Modern Medicine 2014;104-6.
- Chen C. Effect of transvaginal three-dimensional ultrasound in the diagnosis of cesarean scar pregnancy. Modern Practical Medicine 2017;29:745-6.
- Wang W, Zhou Q, Gong Y, et al. Assessment of Fallopian Tube Fimbria Patency With 4-Dimensional Hysterosalpingo-Contrast Sonography in Infertile Women. J Ultrasound Med 2017;36:2061-9. [Crossref] [PubMed]
- Roman H, Carilho J, Da Costa C, et al. Computed tomography-based virtual colonoscopy in the assessment of bowel endometriosis: The surgeon's point of view. Gynecol Obstet Fertil 2016;44:3-10. [Crossref] [PubMed]
- Li J. Effect of transvaginal color Doppler ultrasound in the diagnosis and treatment of cesarean scar pregnancy. Journal of Clinical Medical Literature 2019;6:169. (Electronic Edition).
(English Language Editor: J. Jones)


