
Predictors of clinical outcome after rotational atherectomy-facilitated percutaneous coronary intervention in hemodialysis patients
Introduction
Patients with maintenance hemodialysis (HD) have a 10 to 20 times higher rate of cardiovascular mortality than the general population (1-3). Uremia-related risk factors in addition to traditional risk factors accelerate arteriosclerosis in HD patients (3); disturbance of calcium-phosphorus homeostasis and uremic toxins may lead to accelerated calcification of arterial media (4).
HD and severe calcification of coronary lesions are high-risk factors for restenosis after percutaneous coronary intervention (PCI), even with use of a drug-eluting stent (DES) facilitated by rotational atherectomy (ROTA) (5-7). In our previous overview report on ROTA-facilitated PCI, HD was found to be significantly associated with major adverse cardiovascular events (MACE) and target lesion revascularization (TLR), with the highest hazard ratio (HR) value (8). However, there are limited and conflicting data regarding independent risk factors of ROTA-PCI outcomes in HD patients (8-10). Since the selection bias for ROTA bailout use and the interventionist’s discretion on an individualize lesion, RCT and even registry or retrospective data about ROTA utility in HD patients is of paucity. ROTA is well known as an effective plaque debulking and modification tool in PCI, but the predictors for MACE in HD patients with ROTA-PCI is still unknown. For better and earlier management of the contributing risk factors, we enrolled HD patients with ROTA-facilitated PCI, and retrospectively evaluated data and compared outcome to identify potential risk factors for MACE. We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/apm-21-1658).
Methods
Patient enrollment and exclusion criteria
During the period from January 2013 to December 2015, a consecutive cohort of patients undergoing maintenance HD owing to end-stage renal failure who were treated with ROTA-facilitated PCI were retrospectively included. The data source was the Sapporo Cardiovascular Clinic (SCVC) database. Exclusion criteria were: (I) incomplete follow-up data; (II) non-HD patients; (III) PCI without ROTA pretreatment; and (IV) temporal bailout HD (Figure 1). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by Sapporo Heart Center Institutional Review Board (No. SCVC20210011) and individual consent for this retrospective analysis was waived.
All patients underwent a routine clinical follow-up at least once within 2 years in the outpatient clinic that involved an interview with the interventionist. The presence of MACE was recorded based on findings at the visit or on a telephone call. Follow-up with coronary computed tomographic angiography (CCTA) was performed routinely within 6–12 months after PCI, and coronary angiogram (CAG) was done for new-onset patient symptoms, evidence of cardiac ischemia, or high index of clinical suspicion for significant coronary disease. Blood samples were drawn before and 12 months after PCI to assess the hemoglobin level and lipid profile.
Definitions
Angiographic success for ROTA + stenting was defined as successful stent delivery and expansion with attainment of ≤20% in-stent residual stenosis of the target lesion in the presence of TIMI (Thrombolysis In Myocardial Infarction) flow grade 3. Angiographic success for ROTA + plain old balloon angioplasty (POBA) and ROTA + drug-coated balloon (DCB) was defined as ≤30% residual diameter stenosis by quantitative coronary angiography with TIMI flow grade 3 and no perforation. Follow-up MACE included all-cause death, hospitalization due to heart failure, definite stent thrombosis (ST), and ischemia-driven TLR. TLR was defined as a repeat intervention within 5 mm proximal or distal to the target lesion previously treated in the index procedure or coronary artery bypass graft surgery (CABG) of a lesion in the same epicardial vessel treated in the index procedure. Calcifications were classified as previously reported as mild with a single image or multiple images of non-linear well‐defined calcium density located on the target lesion, moderate with an image of linear calcium density located on one side of the target lesion that was not visible under detailed fluoroscopic imaging with 15 frame per second X-ray fluoroscopy with or without contrast filling, and severe with a linear calcium density image located on both sides of the target lesion that was visible under detailed fluoroscopic imaging (11,12).
PCI procedure and medication
The PCI strategy and decision to perform ROTA was at the interventionist’s discretion. The general indications for ROTA in HD included: (I) lesions with moderate to severe superficial calcification or fibrous calcification compound, identified using imaging methods such as intravascular ultrasound (IVUS) or optical coherence tomography (OCT); (II) calcified lesions with difficulty in passing devices, such as a balloon catheter, imaging catheter, or stent; (III) residual indentation was identified, even when utilizing high-pressure balloon dilatation; (IV) chronic total occlusion lesions, in which the guidewire has been correctly positioned but low-profile balloons cannot be advanced; (V) selected cases of diffuse in-stent restenosis. The ROTA procedure in the SCVC has been described previously (8). Quantitative coronary angiography (QCA) analysis was performed according to the previously reported method using CAAS software ver. 5.9.1. (Pie Medical Imaging, Maastricht, the Netherlands) (13).
With regard to antiplatelet strategy, dual antiplatelet therapy with aspirin and a P2Y12 receptor inhibitor (ticlopidine, 100 mg twice daily or clopidogrel, 75 mg once daily) was administrated in all cases at least 48 hours before PCI and continued for at least 1 year regardless of DES implantation or POBA alone. Statins as well as other secondary prevention drugs and antihypertensive and hypoglycemic agents were prescribed according to current guidelines.
Statistical analysis
Missing data were not imputed because <1% of data for any predictor variable were missing from the cohort data set. Baseline patient characteristics, angiographic data, and parameters during each procedure were compared between patients in the MACE (+) vs. MACE (−) groups in the cohort. The individual outcomes of MACE and TLR were prespecified end points, presented as Kaplan-Meier percentages. Continuous data are presented as mean ± standard deviation or median (interquartile range) (25th and 75th percentiles), as appropriate, and categorical variables are presented as frequency (%). For continuous variables, the Shapiro-Wilk test was used to test the normal distribution of continuous variables. The Student t-test or U test was used to analyze continuous variables depending on the normality of the data distribution, and the Pearson χ2 test or Fisher’s exact test was used for categorical variables, as appropriate. Univariate and multivariate Cox regression analysis was performed to evaluate the independent predictors of MACE. The results are presented as HR with 95% confidence intervals (CIs). All P values were not adjusted for multiple testing. All analyses were performed using IBM SPSS software version 24.0 (IBM Corp., Armonk, NY, USA). All reported P values are two-sided and P values <0.05 were regarded as statistically significant.
Results
Population demographics
A total of 305 HD patients underwent PCI during the study period, and 138 patients with 179 lesions were eligible to enroll the study; of these, 131 patients initially had ROTA-facilitated PCI and bailout was used in 7 patients owing to devices not crossing the lesion or inadequate balloon dilatation. Clinical follow-up was available in 97.5% of patients. Of the 138 enrolled patients, 61 (44.2%) experienced MACE (Table 1). Patients who developed MACE tended to have higher body mass index [BMI; 23.7 (22.0–26.8) vs. 22.4 (20.5–24.4), P=0.003] and included more men (39.3% vs. 18.2%, P=0.006) when compared with MACE (−) patients; in addition, more MACE (+) patients used insulin (18.0% vs. 6.5%, P=0.036) and had hypertension (96.7% vs. 87.0%, P=0.044) and old myocardial infarction (44.3% vs. 23.4%, P=0.009). Age, HD duration, proportion of current smokers, diabetes mellitus, hyperlipidemia, previous PCI or CABG, and atrial fibrillation were comparable between the two groups. Left ventricular ejection fraction (LVEF), hemoglobin, high density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) and glycated hemoglobin (HbA1c) were not significantly different between the groups.
Table 1
| Variable | MACE (+) (n=61) | MACE (−) (n=77) | P value |
|---|---|---|---|
| Age (y) | 68 (54–78) | 71 (65–76) | 0.176 |
| BMI (kg/m3) | 23.7 (22.0–26.8) | 22.4 (20.5–24.4) | 0.003 |
| Male sex, n (%) | 24 (39.3) | 14 (18.2) | 0.006 |
| Current smoker, n (%) | 5 (8.2) | 12 (15.6) | 0.391 |
| HD duration (m) | 74.0 (48.0–112.0) | 76.0 (30.5–163.5) | 0.822 |
| Diabetes mellitus, n (%) | 33 (54.1) | 40 (51.9) | 0.802 |
| Insulin use, n (%) | 11 (18.0) | 5 (6.5) | 0.036 |
| Hypertension, n (%) | 59 (96.7) | 67 (87.0) | 0.044 |
| Hyperlipidemia, n (%) | 43 (70.5) | 47 (61.0) | 0.247 |
| OMI, n (%) | 27 (44.3) | 18 (23.4) | 0.009 |
| Previous PCI, n (%) | 46 (75.4) | 46 (59.7) | 0.052 |
| Previous CABG, n (%) | 11 (18.0) | 12 (15.6) | 0.702 |
| Atrial fibrillation, n (%) | 5 (8.2) | 2 (2.6) | 0.137 |
| LVEF (%) | 60.0 (48.5–64.7) | 61.5 (54.3–67.7) | 0.096 |
| Admission laboratory data | |||
| Hemoglobin (g/dL) | 11.3 (10.3–12.1) | 11.5 (10.3–12.1) | 0.339 |
| HDL-C (mg/dL) | 37.0 (30.0–48.5) | 40.0 (33.0–52.0) | 0.087 |
| LDL-C (mg/dL) | 81.5 (54.5–112.0) | 77.0 (62.5–96.0) | 0.539 |
| HbA1c (%) | 5.8 (5.3–6.9) | 5.5 (5.1–6.2) | 0.059 |
MACE, major adverse cardiovascular events; BMI, body mass index; HD, hemodialysis; OMI, old myocardial infarction; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft surgery; LVEF, left ventricular ejection fraction; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; HbA1c, glycated hemoglobin.
Lesion and procedural characteristics
Lesion characteristics were compared between MACE (+) and MACE (−) groups (Table 2). Most lesions were type B2 and C lesions, according to the modified American College of Cardiology/American Heart Association classification system. There were more in-stent restenosis (ISR) lesions (50.8% vs. 20.8%, P<0.001) in the MACE (+) group than the MACE (−) group. Moreover, the percentage of target vessel right coronary artery (RCA) was much higher (52.5% vs. 33.8%, P=0.027), but that for LAD was lower (21.3% vs. 48.1%, P=0.001) in the MACE (+) group. Other lesion features, such as lesion angulation, bifurcation, ostial lesion, chronic total occlusion (CTO), and lesion length, were comparable between the groups.
Table 2
| Variable | MACE (+) (n=61) | MACE (−) (n=77) | P value |
|---|---|---|---|
| Target vessel, n (%) | |||
| LMT | 3 (4.9) | 6 (7.8) | 0.497 |
| LAD | 13 (21.3) | 37 (48.1) | 0.001 |
| LCX | 14 (23.0) | 11 (14.3) | 0.189 |
| RCA | 32 (52.5) | 26 (33.8) | 0.027 |
| De novo lesion, n (%) | 30 (49.2) | 61 (79.2) | <0.001 |
| ISR, n (%) | 31 (50.8) | 16 (20.8) | <0.001 |
| Lesion angulation >45°, n (%) | 34 (55.7) | 42 (54.5) | 0.058 |
| Bifurcation lesion, n (%) | 26 (42.6) | 35 (45.5) | 0.739 |
| Ostial lesion, n (%) | 29 (47.5) | 30 (39.0) | 0.312 |
| Aorto-ostial lesion, n (%) | 12 (19.7) | 10 (13.0) | 0.287 |
| CTO, n (%) | 7 (11.5) | 5 (6.5) | 0.302 |
| Lesion length (mm) | 29.0 (18.0–40.0) | 29.8 (20.3–40.6) | 0.327 |
| Reference vessel diameter (mm) | 3.3 (2.6–3.6) | 2.7 (2.4–3.3) | 0.004 |
| Lesion type, n (%) | 0.195 | ||
| A | 0 (0.0) | 0 (0.0) | |
| B1 | 0 (0.0) | 0 (0.0) | |
| B2 | 13 (21.3) | 18 (23.4) | |
| C | 48 (78.7) | 59 (76.6) |
MACE, major adverse cardiovascular events; LMT, left main trunk; LAD, left anterior descending coronary; LCX, left circumflex artery; RCA, right coronary artery; ISR, in-stent restenosis; CTO, chronic total occlusion.
All intervention procedural characteristics are also listed in Table 3. IVUS or OCT was applied during all PCI procedures. Of note, the maximum stent diameter, minimum lumen diameter (MLD) after PCI, and acute lumen gain were larger in the MACE (+) group; however, the stent-to-artery ratio [0.8 (0.0–1.0) vs.1.0 (0.0–1.1), P=0.071] and maximum burr-to-artery ratio [0.5 (0.4–0.7) vs. 0.7 (0.6–0.7), P=0.011] were lower in MACE (+) group than MACE (−) group. The total stent length, number of burrs use (≥2), and number of stents use were similar between the two groups. There was also no difference in the PCI strategy (ROTA + POBA, ROTA + DES, ROTA + DCB) or PCI complications [slow/no flow perforation, burr stuck, residual dissection type (A/B)] between groups.
Table 3
| Variable | MACE (+) (n=61) | MACE (−) (n=77) | P value |
|---|---|---|---|
| ROTA bailout usage | 2 (3.2) | 1 (1.3) | 0.584 |
| Image device, n (%) | 61 (100.0) | 77 (100.0) | >0.999 |
| Fluoroscopy time (min) | 27.0 (18.9–38.0) | 26.5 (18.8–37.1) | 0.973 |
| Maximum stent diameter (mm) | 3.5 (3.0–3.5) | 3.0 (2.5–3.5) | 0.027 |
| Maximum burr size (mm) | 1.8 (1.5–2.0) | 1.8 (1.5–2.0) | 0.896 |
| Maximum burr-to-artery ratio | 0.5 (0.4–0.7) | 0.7 (0.6–0.7) | 0.011 |
| Maximum balloon-to-artery ratio | 1.0 (0.9–1.3) | 1.1 (1.0–1.3) | 0.180 |
| Maximum stent-to-artery ratio | 0.8 (0.0–1.0) | 1.0 (0.0–1.1) | 0.071 |
| Total stent length (mm) | 38 [23–42] | 38 [22–48] | 0.898 |
| No. of burrs used (≥2) | 33 (54.1) | 38 (49.4) | 0.579 |
| No. of stents used | 1 (0–1) | 1 (0–1) | 0.411 |
| PCI strategy | 0.395 | ||
| ROTA+POBA | 21 (32.8) | 19 (24.7) | |
| ROTA+DES | 34 (55.7) | 52 (67.5) | |
| ROTA+DCB | 6 (9.8) | 6 (7.8) | |
| Immediate QCA | |||
| MLD before PCI (mm) | 0.8 (0.5–1.2) | 1.0 (0.7–1.3) | 0.189 |
| %DS before PCI | 69 [61–79] | 61 [54–72] | 0.071 |
| MLD after PCI (mm) | 3.1 (2.4–3.6) | 2.8 (2.3–3.2) | 0.005 |
| %DS after PCI | 7 (−2.8 to 17.8) | 8 (−4.0 to 17.0) | 0.904 |
| Acute gain (mm) | 2.0 (1.6–2.5) | 1.7 (1.4–2.1) | 0.030 |
| PCI complications, n (%) | 0.521 | ||
| Slow/no flow | 0 (0.0) | 0 (0.0) | |
| Perforation | 1 (1.6) | 1 (1.3) | |
| Burr stuck | 1 (1.6) | 0 (0.0) | |
| Residual dissection type (A/B) | 0 (0.0) | 0 (0.0) |
PCI, percutaneous coronary intervention; QCA, quantitative coronary angiography; MACE, major adverse cardiovascular events; ROTA, rotational atherectomy; POBA, plain old balloon angioplasty; DES, drug-eluting stent; DCB, drug-coated balloon; MLD, minimum lumen diameter; DS, diameter stenosis.
Clinical follow-up data
Patients were followed up for 362.50 (243.75, 382.25) days. The cumulative rates of MACE and TLR were 44.2% (61/138) (Figure 2A) and 21.01% (29/138) (Figure 2B), respectively. BMI, male sex, target vessel RCA, ISR, and diameter stenosis before PCI were risk factors, and left anterior descending coronary (LAD) was a protective factor for MACE in the univariate analysis. Furthermore, ISR (HR 3.21, 95% CI: 1.59–6.48, P=0.02) was an independent predictors for MACE, in multivariate regression analysis (Table 4). In addition, we further found that ISR (HR 5.08, 95% CI: 1.78–14.47, P=0.02) and acute lumen gain (HR 1.95, 95% CI: 1.12–3.39, P=0.017) were independent predictors for TLR (Table 5).
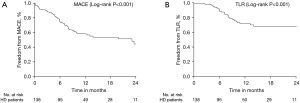
Table 4
| Variable | Univariate analysis, HR (95% CI) | P value | Multivariable analysis, HR (95% CI) | P value |
|---|---|---|---|---|
| PCI strategy | 0.872 | |||
| ROTA+DCB | 1.22 (0.51–2.94) | |||
| ROTA+POBA | 1.10 (0.63–1.91) | |||
| ROTA+DES | 1.00 | |||
| BMI | 1.10 (1.02–1.20) | 0.019 | ||
| Male sex | 2.02 (1.20–3.38) | 0.008 | ||
| Current smoker | 0.73 (0.48–1.10) | 0.128 | ||
| HD duration (m) | 0.99 (0.99–1.00) | 0.060 | ||
| Diabetes | 1.02 (0.61–1.68) | 0.952 | ||
| Statins used | 0.96 (0.56–1.66) | 0.964 | ||
| Insulin used | 1.65 (0.85–3.21) | 0.140 | ||
| Hypertension | 2.74 (0.67–11.26) | 0.161 | ||
| OMI | 1.73 (1.03–2.89) | 0.037 | ||
| Target vessel | ||||
| LAD | 0.38 (0.21–0.71) | 0.002 | 0.31 (0.12–0.76) | 0.011 |
| RCA | 1.99 (1.20–3.30) | 0.008 | ||
| Previous PCI | 1.38 (0.77–2.48) | 0.284 | ||
| LDL-C | 0.99 (0.99–1.01) | 0.853 | ||
| Hemoglobin | 0.88 (0.75–1.03) | 0.109 | ||
| HbA1c | 1.24 (1.01–1.53) | 0.044 | ||
| LVEF | 0.98 (0.86–0.99) | 0.040 | ||
| ISR | 1.90 (1.15–3.15) | 0.013 | 3.21 (1.59–6.48) | 0.001 |
| Contrast volume | 0.99 (0.99–1.00) | 0.093 | ||
| Reference vessel diameter | 1.46 (1.02–2.09) | 0.038 | ||
| Lesion length | 0.99 (0.98–1.01) | 0.44 | ||
| Immediate QCA | ||||
| %DS before PCI | 1.03 (1.01–1.06) | 0.006 | ||
| MLD after PCI | 1.33 (0.83–2.14) | 0.242 | ||
| Acute gain | 1.71 (1.07–2.75) | 0.026 | ||
| Maximum burr-to-artery ratio | 0.29 (0.05–1.54) | 0.146 | ||
| Maximum stent-to-artery ratio | 0.91 (0.57–1.43) | 0.674 |
MACE, major adverse cardiovascular events; PCI, percutaneous coronary intervention; BMI, body mass index; HD, hemodialysis; OMI, old myocardial infarction; LAD, left anterior descending coronary; RCA, right coronary artery; LDL-C, low-density lipoprotein cholesterol; HbA1c, glycated hemoglobin; LVEF, left ventricular ejection fraction; ISR, in-stent restenosis; QCA, quantitative coronary angiography; DS, diameter stenosis; MLD, minimum lumen diameter; HR, hazard ratio; CI, confidence interval.
Table 5
| Variables | Univariate analysis, HR (95% CI) | P value | Multivariable analysis, HR (95% CI) | P value |
|---|---|---|---|---|
| PCI strategy | 0.313 | |||
| ROTA+DCB | 2.21 (0.79–6.13) | |||
| ROTA+POBA | 1.24 (0.56–2.73) | |||
| ROTA+DES | 1.00 | |||
| BMI | 1.12 (0.99–1.25) | 0.053 | ||
| Male sex | 1.43 (0.68–3.05) | 0.348 | ||
| Current smoker | 0.91 (0.55–1.51) | 0.712 | ||
| HD duration (m) | 0.99 (0.99–1.00) | 0.268 | ||
| Diabetes | 1.41 (0.68–2.91) | 0.352 | ||
| Statins used | 1.64 (0.80–3.38) | 0.180 | ||
| Insulin used | 2.86 (1.26–6.49) | 0.012 | ||
| Hypertension | 23.38 (0.09–5,989.25) | 0.265 | ||
| OMI | 2.20 (1.08–4.49) | 0.030 | ||
| Target vessel | ||||
| LAD | 0.25 (0.09–0.65) | 0.004 | 0.29 (0.08–0.99) | 0.049 |
| RCA | 4.27 (2.00–9.12) | <0.001 | ||
| Previous PCI | 4.33 (1.31–14.27) | 0.016 | ||
| LDL-C | 1.00 (0.99–1.01) | 0.437 | ||
| Hemoglobin | 0.91 (0.72–1.14) | 0.394 | ||
| HbA1c | 1.40 (10.6–1.83) | 0.016 | ||
| LVEF | 0.99 (0.96–1.03) | 0.898 | ||
| ISR | 5.49 (2.45–12.33) | <0.001 | 5.08 (1.78–14.47) | 0.002 |
| Contrast volume | 0.99 (0.98–0.99) | 0.023 | ||
| Reference vessel diameter | 1.80 (1.10–2.94) | 0.019 | ||
| Lesion length | 0.99 (0.97–1.02) | 0.493 | ||
| Immediate QCA | ||||
| %DS before PCI | 1.05 (1.01–1.08) | 0.005 | ||
| MLD after PCI | 3.08 (1.47–6.44) | 0.003 | ||
| Acute gain | 3.54 (1.98–6.35) | <0.001 | 1.95 (1.12–3.39) | 0.017 |
| Maximum burr-to-artery ratio | 0.13 (0.01–1.80) | 0.128 | ||
| Maximum stent-to-artery ratio | 0.69 (0.36–1.32) | 0.262 |
TLR, target lesion revascularization; PCI, percutaneous coronary intervention; BMI, body mass index; HD, hemodialysis; OMI, old myocardial infarction; LAD, left anterior descending coronary; RCA, right coronary artery; LDL-C, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; ISR, in-stent restenosis; QCA, quantitative coronary angiography; DS, diameter stenosis; MLD, minimum lumen diameter; HR, hazard ratio; CI, confidence interval.
Because the risk factors related to outcome may be different for de novo and ISR lesions, we stratified the sub-analysis. For de novo lesions, male sex (HR 3.71, 95% CI: 1.66–8.29, P=0.001), target vessel RCA (HR 2.83, 95% CI: 1.28–6.28, P=0.010) and LVEF (HR 0.95, 95% CI: 0.92–0.98, P=0.008) were risk factors for MACE. However, for ISR lesions, only %DS before PCI was an independent risk factor for MACE in the multivariate regression analysis (Table 6).
Table 6
| Variables | De novo | ISR | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis, HR (95% CI) | P value | Multivariable analysis, HR (95% CI) | P value | Univariate analysis, HR (95% CI) | P value | Multivariable analysis, HR (95% CI) | P value | ||
| PCI strategy | 0.521 | 0.802 | |||||||
| ROTA+DCB | – | 0.72 (0.26–1.98) | |||||||
| ROTA+POBA | 1.34 (0.54–3.30) | 0.95 (0.43–2.10) | |||||||
| ROTA+DES | 1.00 | 1.00 | |||||||
| BMI | 1.05 (0.93–1.19) | 0.387 | 1.18 (1.03–1.35) | 0.014 | |||||
| Male | 2.56 (1.23–5.33) | 0.012 | 3.71 (1.66–8.29) | 0.001 | 1.78 (0.84–3.78) | 0.133 | |||
| Current smoker | 0.71 (0.41–1.22) | 0.218 | 1.37 (0.70–2.68) | 0.353 | |||||
| HD duration (m) | 0.99 (0.99–1.00) | 0.212 | 0.99 (0.99–1.00) | 0.086 | |||||
| Diabetes | 0.51 (0.24–1.10) | 0.082 | 2.09 (0.96–4.57) | 0.058 | |||||
| Statins used | 0.94 (0.42–2.13) | 0.890 | 0.84 (0.41–1.77) | 0.661 | |||||
| Insulin used | 0.46 (0.06–3.36) | 0.441 | 2.56 (1.13–5.76) | 0.023 | |||||
| Hypertension | 1.72 (0.41–7.25) | 0.458 | 21.73 (0.01–75,101.27) | 0.458 | |||||
| OMI | 2.06 (0.98–4.32) | 0.056 | 1.23 (0.60–2.51) | 0.573 | |||||
| Target vessel | |||||||||
| LAD | 0.27 (0.11–0.67) | 0.005 | 0.80 (0.34–1.88) | 0.612 | |||||
| RCA | 2.42 (1.16–5.04) | 0.018 | 2.83 (1.28–6.28) | 0.010 | 1.25 (0.61–2.58) | 0.541 | |||
| Previous PCI | 1.17 (0.56–2.46) | 0.670 | 0.57 (0.17–1.93) | 0.367 | |||||
| LDL-C | 0.99 (0.98–1.01) | 0.892 | 0.99 (0.98–1.01) | 0.230 | |||||
| Hemoglobin | 0.81 (0.62–1.06) | 0.125 | 0.96 (0.79–1.16) | 0.671 | |||||
| HbA1c | 1.16 (0.78–1.73) | 0.462 | 1.25 (0.96–1.62) | 0.098 | |||||
| LVEF | 0.96 (0.93–0.99) | 0.019 | 0.95 (0.92–0.98) | 0.008 | 0.99 (0.96–1.02) | 0.680 | |||
| Contrast volume | 0.99 (0.99–1.00) | 0.356 | 0.99 (0.99–1.00) | 0.194 | |||||
| Reference vessel diameter | 1.58 (0.94–2.65) | 0.082 | (–) | 0.320 | |||||
| Lesion length | 0.99 (0.97–1.02) | 0.864 | 0.99 (0.97–1.03) | 0.912 | |||||
| Immediate QCA | |||||||||
| %DS before PCI | 1.01 (0.97–1.05) | 0.522 | 1.05 (1.02–1.08) | 0.004 | 1.05 (1.02–1.08) | 0.004 | |||
| MLD after PCI | 1.27 (0.61–2.65) | 0.522 | 1.21 (0.69–2.11) | 0.498 | |||||
| Acute gain | 1.59 (0.67–3.78) | 0.292 | 1.47 (0.86–2.52) | 0.155 | |||||
| Maximum burr-to-artery ratio | 0.08 (0.01–1.20) | 0.068 | 0.45 (0.09–2.25) | 0.331 | |||||
| Maximum stent-to-artery ratio | 1.33 (0.62–2.87) | 0.463 | 1.08 (0.54–2.15) | 0.819 | |||||
MACE, major adverse cardiovascular events; ISR, in-stent restenosis; PCI, percutaneous coronary intervention; BMI, body mass index; HD, hemodialysis; OMI, old myocardial infarction; LAD, left anterior descending coronary; RCA, right coronary artery; LDL-C, low-density lipoprotein cholesterol; HbA1c, glycated hemoglobin; LVEF, left ventricular ejection fraction; QCA, quantitative coronary angiography; DS, diameter stenosis; MLD, minimum lumen diameter; HR, hazard ratio; CI, confidence interval.
Although the proportion of POBA in the MACE (+) group was slightly higher than the MACE (−) group (Table 3), the PCI strategy was not associated with the studied outcomes with univariate analysis (Tables 4-6). Sensitivity analysis was conducted to furtherly adjust the PCI strategy in the multivariate models, and showed similar finding with the original results (Tables S1-S3), in spite of fluctuation in the confidence intervals due to small sample size.
Because the target vessel location is an important factor for adverse events, we compared the clinical and lesion characteristics between different target vessels. The proportion of RCA location (42.0%, 58/138) was the highest in all target vessels. There were more lesions with angulation >45° but less with bifurcation among RCA than LAD and LCX. The reference vessel diameter was larger in RCA (3.2±0.5 mm) than LAD (2.7±0.6 mm) and LCX (2.7±0.8 mm) (P<0.001) (Table 7).
Table 7
| Variables | LAD (n=50) | RCA (n=58) | LCX (n=25) | P value* |
|---|---|---|---|---|
| Age (y), mean ± SD | 71.0±10.4 | 66.0±11.8 | 67.1±13.5 | 0.161 |
| BMI (kg/m3), mean ± SD | 22.5±3.5 | 23.5±3.3 | 24.2±3.8 | 0.234 |
| De novo lesion, n (%) =0 | 39 (78.0) | 32 (55.2) | 17 (68.0) | 0.043 |
| Lesion angulation >45°, n (%) | 4 (8.0) | 27 (46.6) | 6 (24.0) | <0.001 |
| Bifurcation lesion, n (%) | 32 (64.0) | 8 (13.8) | 18 (72.0) | <0.001 |
| Ostial lesion, n (%) | 24 (48.0) | 19 (32.8) | 14 (56.0) | 0.094 |
| Aorta-ostial lesion, n (%) | 6 (12.0) | 15 (25.9) | 0 (0.0) | 0.008 |
| CTO, n (%) | 1 (2.0) | 7 (12.1) | 4 (25.0) | 0.076 |
| Lesion length (mm), mean ± SD | 32.6±16.8 | 33.2±12.7 | 25.6±7.5 | 0.160 |
| Reference vessel diameter (mm), mean ± SD | 2.7±0.6 | 3.2±0.5 | 2.7±0.8 | <0.001 |
| POBA | 21 (42.0) | 17 (29.3) | 12 (48.0) | 0.196 |
| DES | 28 (56.0) | 41 (70.7) | 13 (52.0) | 0.160 |
| DCB | 3 (6.0) | 8 (13.8) | 1 (4.0) | 0.231 |
| Acute gain | 1.7±0.5 | 2.0±0.7 | 1.8±0.5 | 0.131 |
*, no statistical analysis was performed due to small sample size of left main trunk group (n=5). LAD, left anterior descending coronary; RCA, right coronary artery; LCX, left circumflex artery; BMI, body mass index; CTO, chronic total occlusion; POBA, plain old balloon angioplasty; DES, drug-eluting stent; DCB, drug-coated balloon; SD, standard deviation.
Discussion
We had previously found that ROTA was beneficial for an excessive amount of calcified and fibrotic plaque, and HD was the most significantly related risk factor for cardiac events and TLR in patients undergoing PCI, even facilitated with ROTA (8,14). Furthermore, the present paper demonstrated that the risk of MACE remained high after PCI for calcified lesions in HD patients, even when facilitated with ROTA. TLR contributed half of the MACE in these patients. ISR lesion while RCA in de novo lesion were the main risk factors for MACE in multivariate analysis.
In SCVC, we have ROTA, directional coronary atherectomy (DCA), excimer laser coronary angioplasty (ECLA), cutting/soring balloon and high pressure non-compliant balloon for calcium-modification on hand. And we did not have orbital atherectomy or intravascular lithotripsy yet. As the indication mentioned above, rotational atherectomy is the first choice for the calcium-modification technique in severe calcification cases in our routinely clinical practice. For the moderate and severe calcified lesion, ROTA has the three advantages of device passing, creating crack and plaque debulking. DCA was easy to cause hematoma for deep cutting and, ECLA or cutting balloon were not effective in passing and cracking in severe calcification. Patients with HD and ROTA-needed CAD should be followed up carefully because of their poor prognosis. And the MACE and TLR rates were so high in the first year that early screening and treatment of ISR or ischemia was indispensable before ACS attacked. CCTA presents as an accurate and ideal diagnostic method for vessel noninvasive assessment (15), and limited contrast (less than 20 mL) was needed for CCTA in SCVC clinical practice even though contrast dosing was not that influential for HD patients. Therefore, CCTA was positive and necessary for such group of patients.
Patients with end-stage renal failure have more diffuse coronary disease and more vascular calcification (16,17). Therefore, revascularization for HD patients with coronary artery disease is one of the main challenges for interventional cardiologists owing to the complexity of lesions and high occurrence of target vessel failure. Because inadequate dilatation or spiral dissection owing to severe coronary calcification and barotrauma from non-compliant ballooning may account for a higher restenosis rate, severely calcified lesions may interfere with the diffusion of more hydrophilic antiproliferative drugs in patients undergoing HD. We assumed that superficial calcification debulking would allow for more effective revascularization in HD patients, and the threshold of ROTA use in these patients is relatively low in our center. For sufficient dilatation, the purpose of ROTA here was plaque “modification” and “debulking” as well. An aggressive ablation strategy (higher burr/vessel ratio and low speed polishing) with IVUS guidance can safely and effectively guarantee a smooth and dilatable lumen. In the present cohort, we found that ROTA-facilitated PCI was safe, with a relatively high success rate. However, the midterm outcome was still unfavorable, especially for ISR and TLR. In line with our finding, the J2T ROTA registry, which comprises data from a multicenter, retrospective, cohort study that involved 1,090 patients who underwent ROTA for de novo calcified coronary lesions between 2004 and 2015 in Japan, also found that ROTA-facilitated PCI was associated with rates of MACE as high as 66.9%. The rates of MACE, any death, TLR, and stroke were significantly higher in the HD group than in the non-HD group in the J2T ROTA registry (18). In concern with the PCI strategy, the current concept recommended stenting (including for ISR) unless the patient already had two layers of stent. In our study, 66.7% (92/138) patients had previous stenting PCI and 34.1% (47/138) patients had ISR lesions. We tended to performed POBA or DCB for previous PCI patients, especially for those with long term dual antiplatelet drugs intolerance. Therefore, the frequency of patients receiving POBA and DCB was relatively high in the studied cohort, with a rate of 30% (40/138) and 8.7% (12/138), respectively.
ISR commonly occurs after coronary stent implantation in patients undergoing HD (19). Patients receiving HD tend to have greater calcification of their coronary arteries, which hampers optimization of PCI results (20) and leads to inappropriate vascular healing (21). In contrast to angioplasty, where restenosis is predominantly caused by elastic recoil and vascular remodeling, ISR is almost exclusively owing to neointimal hyperplasia (21,22). Although the optimal treatment of ISR has not yet been well defined, three treatment approaches are commonly used: (I) POBA; (II) atheroablation (ROTA, excimer laser angioplasty and directional coronary atherectomy); and (III) additional stenting. Adamian et al. reported a matched comparison of these three approaches and found that cutting balloon angioplasty was associated with immediate results similar to atheroablation and better clinical and angiographic outcomes at follow-up (23). However, the main application of a cutting balloon is in non-calcified lesions with concentric plaques. In one study, intravascular imaging evaluation (IVUS/OCT) demonstrated that the prevalence of in‐stent calcification was also significantly higher in the HD group compared to the non-HD group (75% versus 5%, respectively; P<0.01), despite the time to ISR being shorter in the HD group (median 10.5 versus 23 months; P=0.13). However, the prevalence of in‐stent lipid‐rich plaque was significantly lower in the HD group (0% versus 43%, respectively; P=0.03) (24). The calcified character of ISR in HD patients poses a great challenge in treatment. In a previous series, we found that ROTA-facilitated PCI was a safe and efficient technique for the treatment of ISR but was still related to a high MACE rate (25). Further, in our present data of HD patients, ISR lesions had an even worse outcome than de novo lesions. The rate of MACE after intervention for ISR was 21.01%, even lower than those in previous reports (26,27).
We found that larger acute gain was related to a higher TLR rate, which indicated that aggressive ROTA may not always be beneficial. Additionally, with the risk of coronary artery perforation, larger acute gain may further injure the intima and media. It has been found that media tears are associated with increased neointima growth (28,29). Farb et al. reported that arterial medial fracture was associated with a 29% increase in neointimal thickness compared with arteries that have an intact media in bare metal stents (28). Moreover, neointimal thickness, degree of inflammation, and neovascularization are greater when struts are in contact with a torn media/intima as compared with struts on an intact fibrous cap. The ideal solution to balance the goals of reducing artery fracture with encouraging strut coverage in heavily calcified lesions may depend on developing atherectomy devices that are able to fracture calcium but not cause excessive injury to the arterial wall. Newer approaches such as a lithotripsy may be promising in this regard, but their advantages still need to be proven against more traditional atherectomy methods (21).
RCA lesion was also an independent risk factor for MACE in de novo lesion, which could be explained by the frequent existence of calcified nodule, the hinge motion or excessive torsion stress this vessel, as well as the presence of ISR lesion. As reported by Morofuji et al. (30), HD patients tended to have calcified nodule in the coronary. Other studies reported calcified nodule is most frequently observed in the RCA (31), and well known to cause poor outcome. However, the lacking of detail information about calcified nodule limited further validation of this hypothesis in the current study. Besides, previous studies showed that hinge motion or excessive torsion stress in RCA is the maximal during the cardiac cycle (31-33). Furthermore, in the current study, RCA had 42.0% of the target lesion located in this vessel, and half of which was ISR lesion. As we known, ISR lesion revascularization was highly associated with poor outcome.
Study limitations
Some limitations should be acknowledged. First, the choice of ROTA was based on the interventionist’s discretion, which would inevitably lead to selection bias. Second, interventional coronary angiography driven by clinical symptoms would also numerically decrease the MACE prevalence owing to the misdiagnosis of silent ischemia. Third, our sample size was relatively small.
Conclusions
In this study, we found that the overall prognosis of ROTA-facilitated PCI in patients receiving HD was very poor, with high mortality. TLR was the predominant major adverse event in ROTA-facilitated PCI among HD patients, even with a high rate of successful procedure. ISR lesion was the main risk factor for MACE in multivariate analysis. And patients with RCA target vessel were more likely to have MACE in de novo lesion.
Acknowledgments
The authors appreciate the contribution of Professor Jiyan Chen and the cooperation of the Sapporo Cardiovascular Clinic and Guangdong Provincial People’s Hospital. We thank LetPub (
Funding: This research was funded and supported by the National Key Research and Development Program of China (grant no. 2016YFC1301202).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/apm-21-1658
Data Sharing Statement: Available at https://dx.doi.org/10.21037/apm-21-1658
Peer Review File: Available at https://dx.doi.org/10.21037/apm-21-1658
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apm-21-1658). HD, ZN and GL report that the funding from National Key Research and Development Program of China (grant no. 2016YFC1301202) supported the study, and the cooperation of the Sapporo Cardiovascular Clinic and Guangdong Provincial People’s Hospital finished the study. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by Sapporo Heart Center Institutional Review Board (No. SCVC20210011) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mahmoodi BK, Matsushita K, Woodward M, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without hypertension: a meta-analysis. Lancet 2012;380:1649-61. [Crossref] [PubMed]
- de Jager DJ, Grootendorst DC, Jager KJ, et al. Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA 2009;302:1782-9. [Crossref] [PubMed]
- Modi ZJ, Lu Y, Ji N, et al. Risk of Cardiovascular Disease and Mortality in Young Adults With End-stage Renal Disease: An Analysis of the US Renal Data System. JAMA Cardiol 2019;4:353-62. [Crossref] [PubMed]
- Chue CD, Townend JN, Steeds RP, et al. Arterial stiffness in chronic kidney disease: causes and consequences. Heart 2010;96:817-23. [Crossref] [PubMed]
- Zheng H, Xue S, Lian F, et al. Meta-analysis of clinical studies comparing coronary artery bypass grafting with percutaneous coronary intervention in patients with end-stage renal disease. Eur J Cardiothorac Surg 2013;43:459-67. [Crossref] [PubMed]
- Otaki Y, Ashikaga T, Sasaoka T, et al. Long-term clinical outcomes of permanent-polymer everolimus-eluting stent implantation following rotational atherectomy for severely calcified de novo coronary lesions: Results of a 22-center study (Tokyo-MD PCI Study). Cardiovasc Revasc Med 2019;20:120-5. [Crossref] [PubMed]
- Tian W, Lhermusier T, Minha S, et al. Rational use of rotational atherectomy in calcified lesions in the drug-eluting stent era: Review of the evidence and current practice. Cardiovasc Revasc Med 2015;16:78-83. [Crossref] [PubMed]
- Dong H, Hachinohe D, Nie Z, et al. Reappraisal Value of a Modified Rotational Atherectomy Technique in Contemporary Coronary Angioplasty Era. J Interv Cardiol 2020;2020:9190702 [Crossref] [PubMed]
- Herzog CA, Ma JZ, Collins AJ. Comparative survival of dialysis patients in the United States after coronary angioplasty, coronary artery stenting, and coronary artery bypass surgery and impact of diabetes. Circulation 2002;106:2207-11. [Crossref] [PubMed]
- Fam JM, Khoo CY, Lau YH, et al. Age and diabetes mellitus associated with worse outcomes after percutaneous coronary intervention in a multi-ethnic Asian dialysis patient population. Singapore Med J 2021;62:300-4. [Crossref] [PubMed]
- Dahdouh Z, Roule V, Dugué AE, et al. Rotational atherectomy for left main coronary artery disease in octogenarians: transradial approach in a tertiary center and literature review. J Interv Cardiol 2013;26:173-82. [Crossref] [PubMed]
- Moussa I, Ellis SG, Jones M, et al. Impact of coronary culprit lesion calcium in patients undergoing paclitaxel-eluting stent implantation (a TAXUS-IV sub study). Am J Cardiol 2005;96:1242-7. [Crossref] [PubMed]
- Lansky AJ, Dangas G, Mehran R, et al. Quantitative angiographic methods for appropriate end-point analysis, edge-effect evaluation, and prediction of recurrent restenosis after coronary brachytherapy with gamma irradiation. J Am Coll Cardiol 2002;39:274-80. [Crossref] [PubMed]
- Hachinohe D, Kashima Y, Kanno D, et al. Rotational atherectomy and new-generation drug-eluting stent implantation. Catheter Cardiovasc Interv 2018;91:1026-34. [Crossref] [PubMed]
- Ru L, Lan P, Xu C, et al. The value of coronary CTA in the diagnosis of coronary artery disease. Am J Transl Res 2021;13:5287-93. [PubMed]
- Charytan D, Kuntz RE, Mauri L, et al. Distribution of coronary artery disease and relation to mortality in asymptomatic hemodialysis patients. Am J Kidney Dis 2007;49:409-16. [Crossref] [PubMed]
- Tsai TT, Messenger JC, Brennan JM, et al. Safety and efficacy of drug-eluting stents in older patients with chronic kidney disease: a report from the linked CathPCI Registry-CMS claims database. J Am Coll Cardiol 2011;58:1859-69. [Crossref] [PubMed]
- Jujo K, Otsuki H, Tanaka K, et al. Long-term cardiovascular prognosis after rotational atherectomy in hemodialysis patients: Data from the J2T multicenter registry. Int J Cardiol 2019;285:14-20. [Crossref] [PubMed]
- Sarnak MJ, Amann K, Bangalore S, et al. Chronic Kidney Disease and Coronary Artery Disease: JACC State-of-the-Art Review. J Am Coll Cardiol 2019;74:1823-38. [Crossref] [PubMed]
- Stenvinkel P, Pecoits-Filho R, Lindholm B. Coronary artery disease in end-stage renal disease: no longer a simple plumbing problem. J Am Soc Nephrol 2003;14:1927-39. [Crossref] [PubMed]
- Torii S, Jinnouchi H, Sakamoto A, et al. Vascular responses to coronary calcification following implantation of newer-generation drug-eluting stents in humans: impact on healing. Eur Heart J 2020;41:786-96. [Crossref] [PubMed]
- Kornowski R, Mintz GS, Kent KM, et al. Increased restenosis in diabetes mellitus after coronary interventions is due to exaggerated intimal hyperplasia. A serial intravascular ultrasound study. Circulation 1997;95:1366-9. [Crossref] [PubMed]
- Adamian M, Colombo A, Briguori C, et al. Cutting balloon angioplasty for the treatment of in-stent restenosis: a matched comparison with rotational atherectomy, additional stent implantation and balloon angioplasty. J Am Coll Cardiol 2001;38:672-9. [Crossref] [PubMed]
- Nakamura N, Torii S, Tsuchiya H, et al. Formation of Calcified Nodule as a Cause of Early In-Stent Restenosis in Patients Undergoing Dialysis. J Am Heart Assoc 2020;9:e016595 [Crossref] [PubMed]
- Hachinohe D, Kashima Y, Hirata K, et al. Treatment for in-stent restenosis requiring rotational atherectomy. J Interv Cardiol 2018;31:747-54. [Crossref] [PubMed]
- Kiriyama H, Kodera S, Minatsuki S, et al. Short-Term and Long-Term Efficacy of Drug-Coated Balloon for In-Stent Restenosis in Hemodialysis Patients with Coronary Artery Disease. Int Heart J 2019;60:1070-6. [Crossref] [PubMed]
- Habara S, Kadota K, Shimada T, et al. Late Restenosis After Paclitaxel-Coated Balloon Angioplasty Occurs in Patients With Drug-Eluting Stent Restenosis. J Am Coll Cardiol 2015;66:14-22. [Crossref] [PubMed]
- Farb A, Weber DK, Kolodgie FD, et al. Morphological predictors of restenosis after coronary stenting in humans. Circulation 2002;105:2974-80. [Crossref] [PubMed]
- Nakano M, Otsuka F, Yahagi K, et al. Human autopsy study of drug-eluting stents restenosis: histomorphological predictors and neointimal characteristics. Eur Heart J 2013;34:3304-13. [Crossref] [PubMed]
- Morofuji T, Kuramitsu S, Shinozaki T, et al. Clinical impact of calcified nodule in patients with heavily calcified lesions requiring rotational atherectomy. Catheter Cardiovasc Interv 2021;97:10-9. [Crossref] [PubMed]
- Virmani R, Kolodgie FD, Burke AP, et al. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol 2000;20:1262-75. [Crossref] [PubMed]
- Lee T, Mintz GS, Matsumura M, et al. Prevalence, Predictors, and Clinical Presentation of a Calcified Nodule as Assessed by Optical Coherence Tomography. JACC Cardiovasc Imaging 2017;10:883-91. [Crossref] [PubMed]
- Torii S, Mustapha JA, Narula J, et al. Histopathologic Characterization of Peripheral Arteries in Subjects With Abundant Risk Factors: Correlating Imaging With Pathology. JACC Cardiovasc Imaging 2019;12:1501-13. [Crossref] [PubMed]



