
Research progress in the pharmacological actions of the multiple effects and selectivity of the vitamin D analogue paricalcitol: a narrative review
Introduction
The discovery of vitamin D (VD) originated from studies on rickets in the early 20th century, and since then, the link between VD and calcium, phosphorus, and bone metabolism has been continuously discovered. Its classical role is to promote intestinal calcium and phosphorus absorption, and then to enter the blood to maintain the balance of calcium and phosphorus levels, as well as to promote bone mineralization and regulate bone homeostasis. Deficiency of VD has now become a global public health problem, with nearly 1 billion people worldwide being in a state of VD insufficiency or deficiency (1).
Vitamin D deficiency is closely related to neuropsychiatric diseases, immunocompromised autoimmune diseases, cardiovascular diseases, joint degeneration osteoarthritis, and allergic diseases (2). Due to the limited intake of vitamin D in food, 90% of vitamin D required by the human body is mainly produced by ultraviolet B (UVB) irradiation of the skin with a wavelength of 280 to 315 nm in sunlight, so insufficient light can cause vitamin D deficiency in the body. Especially with the advancement of industrialization modernization, people’s lifestyles have changed greatly, most of the work and life are carried out indoors, coupled with frequent use of sunscreen clothing and sunscreen, resulting in less and less time for the skin to receive sunlight exposure, leading to vitamin D deficiency increasingly prevalent.
In 2020, the National Health Commission of the People’s Republic of China issued the health industry standard WS/T677-2020 Method for vitamin D deficiency screening of the People’s Republic of China. The standard specifies the indicators, reference judgment values and detection methods for vitamin D deficiency and insufficiency screening in the population. Applicable to the determination of vitamin D nutritional status of the population. At present, vitamin D deficiency can be accurately detected by serum tests (blood alkaline phosphatase, bone alkaline phosphatase) by means of hospital physical examination, and it is also suitable for large-scale screening of vitamin D deficiency.
With the increasing focus on VD deficiency, researchers have found that it is associated with many diseases, and after the 1980s, studies on the non-calcium effects of VD have gradually been conducted. The role of VD in cardiovascular (3), renal (4), bone (5), hematological (6), and autoimmune diseases, and inflammatory reactions (7,8), tumors (9,10), and so on has also been perceived gradually, and subsequently, VD has become a hot spot for clinical and basic research.
In the present study, we summarized the multiple actions and selectivity of paricalcitol, a VD analogue, in multiple organs of the human body. Previously published studies on the molecular mechanism of VD analogues and paricalcitol were analyzed, and current studies on VD analogues were searched for in the PubMed database and database of scientific papers and citations in China. This study aimed to provide a theoretical reference for the pharmacological effects and therapeutic potential of paricalcitol in the field of multiple organ diseases.
We present the following article in accordance with the Narrative Review reporting checklist (available at https://dx.doi.org/10.21037/apm-21-2249).
VD and its analogues
A steroid derivative and a steroid hormone, VD can be consumed from the diet; however, there are few foods which naturally contain VD. The top form of VD is VD2 (ergocalciferol) mainly derived from plants and fungi; VD3 (cholecalciferol) is mainly derived from the fat of deep-sea fish (Figure 1). An interventional study demonstrated (11) that after 2 weeks of administration of the same dose of VD3, VD2, and a placebo to VD deficient individuals, serum 25(OH)D levels were significantly higher in the VD intervention group, but they were significantly higher in those taking VD3 than in the other 2 groups, indicating that the bioavailability of VD3 is superior to that of VD2.
Both VD2 and VD3 are not biologically active on their own and must undergo a series of metabolic processes in the body before they can be converted into their active form (12). Firstly, VD is converted to 25-hydroxyvitamin D [25-(OH)D3] by the 25-hydroxylases CYP2R1 and CYP27A1 in the liver. By 1α-hydroxylase CYP27B1 in the proximal tubule of the kidney, 25-(OH)D3 is then converted to the biologically active 1α, 25-dihydroxyvitamin D3 [1α, 25-(OH)2D3] (13) in the kidney, which enters target cells and binds to the vitamin D receptor (VDR) to play a role in inhibiting parathyroid hormone (PTH) synthesis and regulating calcium, phosphorus, and bone health (14) (Figure 2).
Both VD and 1,25(OH) 2D also have immunomodulatory effects; anti-tumor and anti-leukemia effects by inducing cell differentiation, inhibiting cell proliferation, promoting apoptosis, and inhibiting angiogenesis; renal protective effects by inhibiting renin biosynthesis; stimulating insulin secretion from pancreatic β-cells and improving insulin resistance; and neurological and cardiovascular protective effects (15,16). Therefore, VD supplementation has become an important means of clinical treatment of related diseases.
Currently alfacalcidol, osteopontinol, and dulcolactone are conventional VD drugs used widely in clinical practice as non-selective vitamin D receptor agonists (VDRAs). They present high risks of hypercalcemia, hyperphosphatemia, and metastatic calcification during their use, leading to disturbances in calcium homeostasis in the body, and the eventual outcome of calcium deposition-deposition of calcium and phosphate in soft tissues, especially in vital organs such as the heart and kidneys, as well as in the vascular and respiratory systems, thus limiting their use in clinical practice (17,18). Retaining the A2 ring of non-selective VDRAs binding to VDR, but with appropriate modifications of their D2 ring and its side chains, was shown to significantly improve the selectivity of binding to VDR in the parathyroid glands, with a mechanism of action similar to that of non-selective VDRAs, but with significantly attenuated effects on VDR in other tissues, and the highly selective VD analogue, paricalcitol, was thus created.
New selective VDRA
Paricalcitol, a novel selective VDRA, mainly targets VDR in the parathyroid glands, has less effect on VDR in the intestine and other tissues, inhibits PTH strongly, triggers less hypercalcemia, and has less effect on intestinal absorption of calcium and phosphorus and bone metabolism (19). Paricalcitol inhibits PTH synthesis by binding to VDR in parathyroid cells, and by upregulating parathyroid cell membrane calcium sensing receptor (CaSR) expression, promotes the binding of fibroblast growth factor 23 (FGF23) and fibroblast growth factor receptor 1 (FGFR1), and increases Klotho levels, thereby inhibiting PTH secretion (Figure 3).
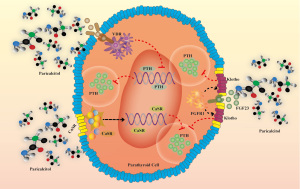
Paricalcitol, as a new VD analogue, has potential side effects as listed in the package insert Adverse Reactions: Approximately 600 patients were treated with HUMIRA in phase II/III/IV clinical studies. Overall, 6% of patients treated with HUMIRA reported adverse reactions. The most common adverse reaction related to HUMIRA treatment was hypercalcemia, which occurred in about 4.7% of patients. Hypercalcemia is related to the degree of oversuppression of PTH, but its risk can be minimized by appropriate dose adjustment.
With the discovery of VDR expression in various different tissues and the revelation of the corresponding physiological effects, a large number of studies have shown that in addition to the treatment of secondary hyperparathyroidism (SHPT), paricalcitol has some therapeutic effects in other conditions such as renal diseases, cardiovascular diseases, immune inflammatory responses, osteoporosis, and tumors (20-22). In this paper, we have focused on an overview of the pharmacological actions of the multiple effects and selectivity of paricalcitol in multiple organs.
Kidney diseases
Currently, the incidence and prevalence of chronic kidney disease (CKD) are increasing annually worldwide, and clinical data show that CKD patients commonly experience relative VD insufficiency or deficiency, even those patients with normal glomerular filtration rate (GFR) indicators (23). There are 2 main therapeutic aspects involved: VD2 or D3 supplementation for 25(OH) D3 deficiency, and 1,25(OH) 2D3 therapy for secondary hyperparathyroidism. The active form of VD not only reduces proteinuria in CKD patients, but also alleviates some complications caused by CKD (24). In all these studies, paricalcitol was shown to be more effective in kidney injury (25).
Active VD and its analogues are commonly used in the treatment of SHPT in hemodialysis patients, and several studies in recent years have shown that treatment with active VD and its analogues can improve VD deficiency and correct SHPT in patients with CKD, reduce mortality, and improve patient prognosis (26).
Although VD replacement therapy is generally effective in reducing PTH levels, it has a negative effect of increase of blood calcium concentration due to its high absorption of calcium ions. Hypercalcemia not only leads to ectopic calcification, but also to excessive suppression of PTH, resulting in osteodystrophy and vascular calcification (27). Therefore, there are some limitations in VDR therapy.
Paricalcitol is the first VD analogue that has been shown to be effective in the treatment of SHPT. In a rat model study of uremia, paricalcitol could effectively inhibit PTH level at all experimental doses (8.0, 25, and 75 ng per rat), with no significant increase in blood calcium and phosphorus in vivo. The 75 ng dose group had the greatest therapeutic effect, with PTH level ranging from 193±49 to 53±16 pg/mL, the total effective rate higher than that of the control group, the parathyroid volume and the incidence of skin pruritus, general fatigue, and musculoskeletal pain were lower than those of the control group (28). In a double-blind, multicenter, randomized controlled clinical trial, the efficacy of paricalcitol (experimental group) and calcitriol (control group) was observed. The results showed that the PTH value of the experimental group decreased by 50% compared with the original, and the medication time of the experimental group was shorter than that of the control group (29). Fernström et al. (30) treated 92 SHPT patients with an average age of 64 years by intravenous injection of paricalcitol for 1 year in Sweden, and the results showed such treatment to be safe and effective. Paricalcitol, while effectively controlling high blood calcium and PTH levels, also inhibits parathyroid hyperplasia, which is associated with its induction of the cell cycle inhibitor p21 and inhibition of transforming growth factor-α (TGF-α) and its receptor (31). Paricalcitol drug experiments by He et al. (32) in 5/6 nephrectomized rats showed that it could inhibit the local expression of the RAS gene and vascular endothelial growth factor (VEGF) in the kidney and significantly reduce the glomerulosclerotic index.
The molecular mechanism of the nephroprotective effect of paricalcitol is related to its inhibition of nuclear factor kappa-B (NF-κB) signaling pathway and inhibition of the transformation process of renal tubular epithelial-mesenchymal transition (EMT). Evidence from Du et al. (33) showed that lipopolysaccharide induced apoptosis in renal tubular cells by upregulating PUMA and miR-155, while paricalcitol protected renal tubular epithelial cells by blocking NF-κB signaling pathway-mediated PUMA and miR-155 protein upregulation. By observing the renal pathological changes and autophagy-related protein expression in diabetic nephropathy (DN) rats with intraperitoneal injection of paricalcitol, Ying et al. (34) found that paricalcitol ameliorated renal injury in DN rats by a mechanism related to elevated tubulointerstitial E-cadherin to alleviate EMT and upregulation of autophagy-related proteins (tubular epithelial Klotho protein, renal cortical LC3-II/LC3- I) expression to enhance autophagy. Studies from Zhang et al. (35) showed that paricalcitol alone for streptozotocin (STZ)-induced DN mice exerted renoprotective effects by inhibiting renin transcription, and the use of paricalcitol in combination with losartan completely avoided proteinuria, repaired the glomerular filtration barrier, and reduced glomerulosclerosis. It has also been shown (36) that paricalcitol increases the expression of Klotho protein in renal tubular epithelial cells. Studies have shown (37) that the treatment regimen of losartan combined with paricalcitol can have a renoprotective effect in rats with DN, reducing the fibrosis of renal tissue and delaying the progression of DN. However, a recent study by Rayego-Mateos et al. (38) demonstrated that paricalcitol exerted anti-inflammatory effects by regulating tumor necrosis factor (TNF) receptor-associated factor 3 (TRAF3) and inhibiting non-classical NF-κB signaling pathway, which may be a new mechanism of renal protection.
In summary, paricalcitol can inhibit the classical NF-κB signaling pathway and exerts anti-inflammatory and anti-fibrotic effects by regulating TRAF3 to inhibit the non-classical NF-κB signaling pathway, and may also enhance intracellular autophagy in renal tubular epithelial cells by upregulating Klotho autophagy signaling activity, thereby inhibiting tubular EMT, reducing proteinuria, improving renal function, alleviating renal fibrosis, and slowing the progression of renal failure (Figure 4).
Cardiovascular diseases
In recent years, epidemiological and clinical studies have shown that VD might be involved in cardiovascular protection by affecting or regulating inflammatory cytokines (39), vascular calcification (40), and the renin-angiotensin-aldosterone system (41). Deficiency of VD can cause increased risk of cardiovascular diseases, including atherosclerosis. Studies have reported that the deficiency of VD in vivo is related to the onset of coronary heart diseases (42). Low VD levels are associated with atherosclerosis (43,44), coronary artery disease, myocardial infarction (45), heart failure (46), stroke (47), cardiovascular mortality (48), and all-cause mortality, and are independent risk factors for cardiovascular diseases (49).
The results of in vitro experiments on neonatal rat cardiomyocytes (50) and in vivo experiments on rats with kidney disease (51) have shown that paricalcitol can significantly slow the process of left ventricular hypertrophy (52), promote the absorption of blood calcium in the gastrointestinal tract, and stimulate the secretion of calcitonin, thereby improving bone tissue reconstruction and inhibiting the calcification of peripheral tissues such as blood vessels (53). It was revealed that upregulation of VDR expression by paricalcitol might be one of the reasons for its efficacy in improving left ventricular hypertrophy, and alleviating myocardial weakness and myocardial compression.
Tamayo et al. (54) showed that in patients with heart failure, paricalcitol can prevent the disease progress, improve the electrophysiological and calcium disorders, and reduce the susceptibility to heart failure-related ventricular arrhythmias. It may be a potential treatment option for heart failure. In an amitriptyline-induced cardiotoxicity experiment in rats (55), electrocardiogram (ECG) showed abnormalities, cardiac troponin T (cTnT) level was increased, and paricalcitol treatment could reverse the cardiotoxicity in rats. In addition, when combined with enalapril, paricalcitol can effectively reduce the expression of inflammatory factors and oxidative factors, and prevent the occurrence of oxidative damage and inflammation in ApoE-/- mice (56). Becker et al. (57) studied the effect of paricalcitol and calcitriol on the structure of the arterial wall of ApoE-/- mice after unilateral nephrectomy and found that the arterial wall of mice treated with calcitriol had obviously thickened, but there was no such side effect after treatment with paricalcitol. In hypertensive patients with CKD stage 3–5 and secondary hyperparathyroidism, arterial stiffness was improved and carotid femoral pulse wave velocity (PWV) was decreased after treatment with paricalcitol (58).
In summary, paricalcitol can prevent and treat cardiovascular diseases without causing vascular calcification, regulate the adhesion of endothelial cells, and protect endothelial cells from activation, so as to reduce the migration and proliferation of vascular smooth muscle cells and the formation of atherosclerotic plaque, and slow down the occurrence and progression of early atherosclerosis (Figure 5).
Bone and muscle diseases
Bone health is closely related to calcium and active VD. Clinically, VD deficiency can significantly reduce muscle strength and motility, cause rickets and osteoporosis, and VD and calcium supplementation can significantly reduce the risk of osteoporotic fractures (59). Molecular medicine studies have pointed out that myofibroblasts contain a large number of VDRs that receive signals from VD hormones. Additionally, VD can increase the troponin complex in muscle tissue, increase the number of muscle fibers, and thicken muscle fibers. Paricalcitol may also improve bone metabolism through other mechanisms in addition to its role in regulating PTH, calcium, and phosphorus metabolism.
Carvalho et al. (60) found that after treatment with paricalcitol in a diabetic zebrafish model, the increase in bone mineralization and regeneration in the experimental group animals could be explained by an increase in osteoblast differentiation and insulin expression, indicating that paricalcitol has the potential to promote bone cell formation. In ex vivo cultures of neonatal mouse skulls, the stimulating effect of paricalcitol on osteoclasts was much lower than that of calcitriol (61). Patients with SHPT are often complicated by abnormal bone metabolism, for which some researchers have suggested that carboxy-terminal parathyroid hormone (C-PTH) is an important indicator of bone transformation inhibition (62) and can inhibit bone metabolism to some extent.
In summary, paricalcitol, on the one hand, can induce the transformation of pre-osteoblasts into osteoblasts. The released osteocalcin reacts with carboxylase to form carboxylated osteocalcin, producing the effect of introducing calcium into bone, and at the same time, it reacts with collagen secreted by osteoblasts to form collagen fibers to promote bone formation. On the other hand, it can inhibit the transformation of pre-osteoclasts into osteoclasts, thus reducing the release of cyclooxygenase (COX-2) and prostaglandin (PGD-2) secreted by osteoclasts, improving the imbalance of bone remodeling, and increasing bone mass (Figure 6).
Immune inflammation
The effects of VD on intrinsic immunity are mainly focused on the regulation of epithelial cells, mononuclear phagocytes, and dendritic cells, with the most studied being the regulation of mononuclear phagocytes. On the one hand, VD enhances bactericidal function by increasing the production of cathelicidin antimicrobial peptide (CAMP) and defensins. On the other hand, VD reduces the severity of infection through reducing the systemic inflammatory response by downregulating the synthesis of several pro-inflammatory cytokines, such as TNF-α, interleukin (IL)-1β, IL-6, IL-8, IL-23, and interferon (IFN)-γ.
Experts in China and internationally have found that VD inhibits dendritic cell maturation and differentiation, reduces the expression of costimulatory molecules and decreases IL-12 production, and regulates and induces T-cell immune tolerance (63). Numerous studies in vitro and in vivo have strongly showed that VD enhances antimicrobial capacity and suppresses immune-mediated inflammatory responses. However, VD application can lead to hypercalcemia, which is the biggest limitation for the treatment of inflammation.
The immunomodulatory potential of paricalcitol enables it to be a therapeutic drug for chronic immune-mediated inflammatory diseases (64). A large number of studies have found that paricalcitol has a significant effect in maintaining the balance between oxidation and anti-oxidation (65). In a mouse model of atherosclerosis, paricalcitol was found to have anti-inflammatory effects and oxidative stress regulation effects on the aorta and kidneys (66). In clinical trials, Navarro-González et al. found that paricalcitol significantly increased the levels of superoxide dismutase (SOD), catalase (CAT), reduced glutathione (GSH), thioredoxin (TRX), and other antioxidants in dialysis patients (67). Eleftheriadis et al. discovered via in vitro experiments that paricalcitol can significantly reduce the levels of TNF-α, IL-8, C-reactive protein (CRP), and other inflammatory cytokines, and significantly increase the level of anti-inflammatory cytokine IL-10 (68). Similar results were found in a study of Chinese hemodialysis patients with SHPT in 2018. After 2 months of treatment with paricalcitol, CRP, IL-6, IL-8, and TNF-a all decreased significantly compared with before (69-71). These findings confirmed that the selective VD receptor agonist paricalcitol also had the functions of reducing pro-inflammatory cytokines and promoting anti-inflammatory cytokines, and can play a role in balancing the excessive immune inflammatory response.
In summary, paricalcitol may reduce the production of reactive oxygen species (ROS) by down-regulating the renin-angiotensin-aldosterone system, thereby reducing the production and release of inflammatory cytokine TNF-α and other inflammatory mediators, as well as the activation of inflammatory regulatory factors such as transcription factor NF-κB, thereby reducing the infiltration of inflammatory cells and the damage to heart and kidney (Figure 7).
Anti-tumor effect
A large number of epidemiological studies have found that VD and its analogues play an important role in the prevention and treatment of a variety of tumors. Many researchers have studied the mechanism of this action from a molecular perspective and found that VD can prevent and treat cancer through cell cycle arrest, induction of apoptosis, and regulation of growth factors, among others. It has been shown that paricalcitol has a tumor inhibitory effect similar to that of VD (72).
Researchers at the Salk Institute For Biological Studies found that (73) paricalcitol, an analogue of VD, can break through the protective matrix around tumor cells. Gombart et al. (74) showed that paricalcitol can inhibit the proliferation and angiogenesis of breast cancer, with good tolerance, without grade 2 or higher hypercalcemia, which is safe and feasible for breast cancer patients undergoing chemotherapy.
Schwartz et al. (75) found that paricalcitol is an active form of VD, which has the effects of inhibiting, promoting apoptosis, and anti-metastasis of tumor cells in vivo and in vivo. Therefore, the inhibition of paricalcitol on the growth of pancreatic cancer cell lines in vitro and in vivo was studied, and the results showed that paricalcitol inhibited the growth of BXPC-3, Hs700t, and ASPC-1 pancreatic cancer cells, and was dose-dependent. This anti-proliferative activity is related to the up-regulation of cell cycle inhibitors p21 (WaF1/CIP1) and p27 (Kip1).
The combined use of paricalcitol and arsenic trioxide (As2O3) has a strong anti-proliferative effect on acute myeloid leukemia cells, and can induce the significant differentiation of mononuclear cells of acute promyelocytic leukemia cell line NB-4 and acute myeloid leukemia cell line HL-60, which in turn induces apoptosis by reducing Bcl-2 and Bcl-xL protein levels (76).
Paricalcitol can synergize calcitriol in the radiotherapy of prostate cancer, better inhibiting the proliferation in vitro of human prostate cancer cells and promoting cell apoptosis. Another clinical study showed that none of the 18 patients with stage I/II prostate cancer had prostate-specific antigen reactions after treatment with paricalcitol, and the PTH level decreased in 7 patients, which supported the safety of paricalcitol in the treatment of prostate cancer.
In summary, paricalcitol may play an anti-tumor role by breaking through the protective matrix surrounding tumor cells, increasing T cell penetration into the tumor, inhibiting tumor cell proliferation, inducing cell differentiation, promoting cell apoptosis, inhibiting angiogenesis, inhibiting tumor cell infiltration and metastasis, and inhibiting inflammation (Figure 8).
Conclusions
The novel VD analogue, paricalcitol, with its high selectivity for binding to VDR in vivo, maintains the efficacy of traditional VD drugs (targeting PTH and calcium and phosphorus metabolism) while providing additional benefits (reduction of urinary protein, reduction of inflammation, reduction of vascular calcification and renal fibrosis, and so on), thus expanding the application scope of future clinical practice; however, there are also some controversies and problems [the effects on estimated (e)GFR, the increase of cardiovascular and death risks in the long term, the effect on sugar metabolism, and so on] which need further attention and research.
With more understanding and application of the effects of active VD and analogues, scientists in China and worldwide are more committed to investigating the mechanism of action of paricalcitol in anti-inflammation, immunomodulation, and anti-proliferation. We believe that through further trials and studies, more evidence-based medical research will be carried out to provide a deeper and broader understanding of the molecular mechanisms of paricalcitol, allowing it to exert greater therapeutic effects and clinical benefits.
Acknowledgments
The authors would like to thank International Science Editing (
Funding: This study was supported by Xinjiang Uygur Autonomous Region Science and Technology Project, China (2020E02118).
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dx.doi.org/10.21037/apm-21-2249
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apm-21-2249). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shanshan J, Shuguang S. Research Progress of Vitamin D. Medical Innovation of China 2020;17:167-72.
- Chang SW, Lee HC. Vitamin D and health-The missing vitamin in humans. Pediatr Neonatol 2019;60:237-44. [Crossref] [PubMed]
- Jianhua Q, Li W. Research Progress on the relationship between Vitamin D deficiency and risk factors of Cardiovascular Disease. Chinese Journal of Clinicians 2016;10:279-83.
- Zoccali C, Mallamaci F. Moderator's view: Vitamin D deficiency treatment in advanced chronic kidney disease: a close look at the emperor's clothes. Nephrol Dial Transplant 2016;31:714-6. [Crossref] [PubMed]
- Huijuan X, Shengqiang L, Li H, et al. Correlation between kidney yin deficiency syndrome and expression of immune-related genes LRG1 and SRC mRNA in postmenopausal women with osteoporosis. Chinese Journal of traditional Chinese Medicine 2017;32:1347-50.
- Wei J, Fengrong G. Research progress on diseases related to vitamin D deficiency. Chinese Journal of Osteoporosis 2014;20:331-7.
- Tural Onur S, Yalcin AD, Celik B, et al. Evaluation of d-dimer, CXCL8, homocysteine, eosinophil cationic peptide, 25(OH)-vitamin D and immunomodulatory OX-2 levels in allergic patients. J Asthma 2015;52:123-7. [Crossref] [PubMed]
- Bartley J, Garrett J, Grant CC, et al. Could vitamin d have a potential anti-inflammatory and anti-infective role in bronchiectasis? Curr Infect Dis Rep 2013;15:148-57. [Crossref] [PubMed]
- Mian L, Peizhan C, Xiaoguang L, et al. Vitamin D and Cancer: research status and Prospect. Life Science 2013;25:218-30.
- Driel MV, Leeuwen JPTMV, Muoz A, et al. Chapter 94-Overview of vitamin D actions in cancer. Vitamin D 2018;1:711-42.
- Yanyan Y. A randomized controlled study of vitamin D2 and vitamin D3 in the treatment of rickets. Grass-roots Medical Forum 2016;20:3493-4.
- Christakos S, Ajibade DV, Dhawan P, et al. Vitamin D: metabolism. Rheum Dis Clin North Am 2012;38:1-11. vii. [Crossref] [PubMed]
- Haussler MR, Whitfield GK, Kaneko I, et al. Molecular mechanisms of vitamin D action. Calcif Tissue Int 2013;92:77-98. [Crossref] [PubMed]
- Dusso AS, Brown AJ, Slatopolsky E, Vitamin D. Am J Physiol Renal Physiol 2005;289:F8-28. [Crossref] [PubMed]
- Bikle DD. What is new in vitamin D: 2006-2007. Curr Opin Rheumatol 2007;19:383-8. [Crossref] [PubMed]
- Leyssens C, Verlinden L, Verstuyf A. The future of vitamin D analogs. Front Physiol 2014;5:122. [Crossref] [PubMed]
- Slatopolsky E, Brown AJ. Vitamin D analogs for the treatment of secondary hyperparathyroidism. Blood Purif 2002;20:109-12. [Crossref] [PubMed]
- Young EW, Albert JM, Satayathum S, et al. Predictors and consequences of altered mineral metabolism: the Dialysis Outcomes and Practice Patterns Study. Kidney Int 2005;67:1179-87. [Crossref] [PubMed]
- Mathew S, Lund RJ, Chaudhary LR, et al. Vitamin D receptor activators can protect against vascular calcification. J Am Soc Nephrol 2008;19:1509-19. [Crossref] [PubMed]
- Goldenberg MM. Paricalcitol, a new agent for the management of secondary hyperparathyroidism in patients undergoing chronic renal dialysis. Clin Ther 1999;21:432-41. [Crossref] [PubMed]
- Cozzolino M, Ketteler M, Zehnder D. The vitamin D system: a crosstalk between the heart and kidney. Eur J Heart Fail 2010;12:1031-41. [Crossref] [PubMed]
- Kovesdy CP, Kalantar-Zadeh K. Vitamin D receptor activation and survival in chronic kidney disease. Kidney Int 2008;73:1355-63. [Crossref] [PubMed]
- Ahmadi AR, Lafranca JA, Claessens LA, et al. Shifting paradigms in eligibility criteria for live kidney donation: a systematic review. Kidney Int 2015;87:31-45. [Crossref] [PubMed]
- Palmer SC, McGregor DO, Macaskill P, et al. Meta-analysis: vitamin D compounds in chronic kidney disease. Ann Intern Med 2007;147:840-53. [Crossref] [PubMed]
- Tian J, Liu Y, Williams LA, et al. Potential role of active vitamin D in retarding the progression of chronic kidney disease. Nephrol Dial Transplant 2007;22:321-8. [Crossref] [PubMed]
- Wei W, Gui S, Li W. Recommended guidelines for active vitamin D and its analogues in the treatment of secondary hyperparathyroidism in hemodialysis patients. Journal of Kidney Disease and Dialysis Kidney Transplantation 2017;26:381-5.
- Hruska KA, Saab G, Mathew S, et al. Renal osteodystrophy, phosphate homeostasis, and vascular calcification. Semin Dial 2007;20:309-15. [Crossref] [PubMed]
- Brown AJ, Slatopolsky E. Drug insight: vitamin D analogs in the treatment of secondary hyperparathyroidism in patients with chronic kidney disease. Nat Clin Pract Endocrinol Metab 2007;3:134-44. [Crossref] [PubMed]
- Abdul Gafor AH, Saidin R, Loo CY, et al. Intravenous calcitriol versus paricalcitol in haemodialysis patients with severe secondary hyperparathyroidism. Nephrology (Carlton) 2009;14:488-92. [Crossref] [PubMed]
- Fernström A, Giæver J, Granroth B, et al. Achievement of recommended treatment targets for bone and mineral metabolism in haemodialysis patients using paricalcitol: an observational study. Scand J Urol Nephrol 2011;45:196-205. [Crossref] [PubMed]
- Xianfu Q. Clinical efficacy and safety of paricalcitol in the treatment of hyperparathyroidism secondary to chronic renal failure. Clinical Rational Use of Drugs 2020;4:56-8.
- He Z, Riva M, Björk P, et al. CD14 Is a Co-Receptor for TLR4 in the S100A9-Induced Pro-Inflammatory Response in Monocytes. PLoS One 2016;11:e0156377 [Crossref] [PubMed]
- Du J, Jiang S, Hu Z, et al. Vitamin D receptor activation protects against lipopolysaccharide-induced acute kidney injury through suppression of tubular cell apoptosis. Am J Physiol Renal Physiol 2019;316:F1068-77. [Crossref] [PubMed]
- Ying L, Junya J, Shan L. Observation on Renal pathological changes and expression of Autophagy-related proteins in Diabetic Nephropathy Rats induced by intraperitoneal injection of paricalcitol. Shandong Medicine 2019;9:48-51.
- Zhang Z, Sun L, Wang Y, et al. Renoprotective role of the vitamin D receptor in diabetic nephropathy. Kidney Int 2008;73:163-71. [Crossref] [PubMed]
- Ritter CS, Zhang S, Delmez J, et al. Differential expression and regulation of Klotho by paricalcitol in the kidney, parathyroid, and aorta of uremic rats. Kidney Int 2015;87:1141-52. [Crossref] [PubMed]
- Ming Z, Yan H, Jia L. Effects of losartan combined with paricalcitol on the expression of CTGF and RhoA/ROCK1 in renal tissue of diabetic rats. Journal of Clinical and Experimental Medicine 2018;17:903-6.
- Rayego-Mateos S, Morgado-Pascual JL, Valdivielso JM, et al. TRAF3 Modulation: Novel Mechanism for the Anti-inflammatory Effects of the Vitamin D Receptor Agonist Paricalcitol in Renal Disease. J Am Soc Nephrol 2020;31:2026-42. [Crossref] [PubMed]
- Zanetti M, Harris SS, Dawson-Hughes B. Ability of vitamin D to reduce inflammation in adults without acute illness. Nutr Rev 2014;72:95-8. [Crossref] [PubMed]
- Hansen D, Rasmussen K, Rasmussen LM, et al. The influence of vitamin D analogs on calcification modulators, N-terminal pro-B-type natriuretic peptide and inflammatory markers in hemodialysis patients: a randomized crossover study. BMC Nephrol 2014;15:130. [Crossref] [PubMed]
- Norman PE, Powell JT. Vitamin D and cardiovascular disease. Circ Res 2014;114:379-93. [Crossref] [PubMed]
- Miao L, Xufeng H, Jun W. A preliminary study on the relationship between vitamin D level and coronary heart disease. Chinese Medicine and Clinic 2011;11:898-9.
- Giovinazzo S, Alibrandi A, Campennì A, et al. Correlation of cardio-metabolic parameters with vitamin D status in healthy premenopausal women. J Endocrinol Invest 2017;40:1337-43. [Crossref] [PubMed]
- Hao Y, Ma X, Luo Y, et al. Additional role of serum 25-hydroxyvitamin D3 levels in atherosclerosis in Chinese middle-aged and elderly men. Clin Exp Pharmacol Physiol 2014;41:174-9. [Crossref] [PubMed]
- Milazzo V, De Metrio M, Cosentino N, et al. Vitamin D and acute myocardial infarction. World J Cardiol 2017;9:14-20. [Crossref] [PubMed]
- Gotsman I, Shauer A, Zwas DR, et al. Vitamin D deficiency is a predictor of reduced survival in patients with heart failure; vitamin D supplementation improves outcome. Eur J Heart Fail 2012;14:357-66. [Crossref] [PubMed]
- Afshari L, Amani R, Soltani F, et al. The relation between serum Vitamin D levels and body antioxidant status in ischemic stroke patients: A case-control study. Adv Biomed Res 2015;4:213. [PubMed]
- Anderson JL, May HT, Horne BD, et al. Relation of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am J Cardiol 2010;106:963-8. [Crossref] [PubMed]
- Pérez-Hernández N, Aptilon-Duque G, Nostroza-Hernández MC, et al. Vitamin D and its effects on cardiovascular diseases: a comprehensive review. Korean J Intern Med 2016;31:1018-29. [Crossref] [PubMed]
- Thadhani R, Appelbaum E, Chang Y, et al. Vitamin D receptor activation and left ventricular hypertrophy in advanced kidney disease. Am J Nephrol 2011;33:139-49. [Crossref] [PubMed]
- Wu-Wong JR, Chen YW, Nakane M, et al. Differential effects of vitamin d receptor agonists on gene expression in neonatal rat cardiomyocytes. Cardiovasc Drugs Ther 2011;25:215-22. [Crossref] [PubMed]
- Mizobuchi M, Nakamura H, Tokumoto M, et al. Myocardial effects of VDR activators in renal failure. J Steroid Biochem Mol Biol 2010;121:188-92. [Crossref] [PubMed]
- Li X, Wei Y, Shao H, et al. Efficacy and safety of microwave ablation for ectopic secondary hyperparathyroidism: a feasibility study. Int J Hyperthermia 2019;36:647-53. [Crossref] [PubMed]
- Tamayo M, Martín-Nunes L, Val-Blasco A, et al. Beneficial effects of paricalcitol on cardiac dysfunction and remodelling in a model of established heart failure. Br J Pharmacol 2020;177:3273-90. [Crossref] [PubMed]
- Aygun H, Basol N, Gul SS. Cardioprotective Effect of Paricalcitol on Amitriptyline-Induced Cardiotoxicity in Rats: Comparison of 99mTcPYP Cardiac Scintigraphy with Electrocardiographic and Biochemical Findings. Cardiovasc Toxicol 2020;20:427-36. [Crossref] [PubMed]
- Husain K, Suarez E, Isidro A, et al. Effects of paricalcitol and enalapril on atherosclerotic injury in mouse aortas. Am J Nephrol 2010;32:296-304. [Crossref] [PubMed]
- Becker LE, Koleganova N, Piecha G, et al. Effect of paricalcitol and calcitriol on aortic wall remodeling in uninephrectomized ApoE knockout mice. Am J Physiol Renal Physiol 2011;300:F772-82. [Crossref] [PubMed]
- Giakoumis M, Tsioufis C, Dimitriadis K, et al. Effects of oral paricalcitol therapy on arterial stiffness and osteopontin in hypertensive patients with chronic kidney disease and secondary hyperparathyroidism. Hellenic J Cardiol 2019;60:108-13. [Crossref] [PubMed]
- Gorter EA, Krijnen P, Schipper IB. Vitamin D deficiency in adult fracture patients: prevalence and risk factors. Eur J Trauma Emerg Surg 2016;42:369-78. [Crossref] [PubMed]
- Carvalho FR, Fernandes AR, Cancela ML, et al. Improved regeneration and de novo bone formation in a diabetic zebrafish model treated with paricalcitol and cinacalcet. Wound Repair Regen 2017;25:432-42. [Crossref] [PubMed]
- Menezes FG, Abreu RM, Itria A. Cost-effectiveness analysis of paricalcitol versus calcitriol for the treatment of SHPT in dialytic patients from the SUS perspective. J Bras Nefrol 2016;38:313-9. [Crossref] [PubMed]
- Moe SM, Saifullah A, LaClair RE, et al. A randomized trial of cholecalciferol versus doxercalciferol for lowering parathyroid hormone in chronic kidney disease. Clin J Am Soc Nephrol 2010;5:299-306. [Crossref] [PubMed]
- Campbell MJ, Adorini L. The vitamin D receptor as a therapeutic target. Expert Opin Ther Targets 2006;10:735-48. [Crossref] [PubMed]
- Müller DN, Kleinewietfeld M, Kvakan H. Vitamin D review. J Renin Angiotensin Aldosterone Syst 2011;12:125-8. [Crossref] [PubMed]
- Mittman N, Desiraju B, Meyer KB, et al. Treatment of secondary hyperparathyroidism in ESRD: a 2-year, single-center crossover study. Kidney Int Suppl 2010;S33-6. [Crossref] [PubMed]
- Husain K, Suarez E, Isidro A, et al. Effect of paricalcitol and enalapril on renal inflammation/oxidative stress in atherosclerosis. World J Biol Chem 2015;6:240-8. [Crossref] [PubMed]
- Navarro-González JF, Donate-Correa J, Méndez ML, et al. Anti-inflammatory profile of paricalcitol in hemodialysis patients: a prospective, open-label, pilot study. J Clin Pharmacol 2013;53:421-6. [Crossref] [PubMed]
- Eleftheriadis T, Antoniadi G, Liakopoulos V, et al. Paricalcitol reduces basal and lipopolysaccharide-induced (LPS) TNF-alpha and IL-8 production by human peripheral blood mononuclear cells. Int Urol Nephrol 2010;42:181-5. [Crossref] [PubMed]
- Lu W, Jiang JP, Hu J, et al. Curcumin protects against lipopolysaccharide-induced vasoconstriction dysfunction via inhibition of thrombospondin-1 and transforming growth factor-β1. Exp Ther Med 2015;9:377-83. [Crossref] [PubMed]
- Rodrigues SD, França KC, Dallin FT, et al. N-acetylcysteine as a potential strategy to attenuate the oxidative stress induced by uremic serum in the vascular system. Life Sci 2015;121:110-6. [Crossref] [PubMed]
- Eelen G, Gysemans C, Verlinden L, et al. Mechanism and potential of the growth-inhibitory actions of vitamin D and ana-logs. Curr Med Chem 2007;14:1893-910. [Crossref] [PubMed]
- Bigelsen S. Evidence-based complementary treatment of pancreatic cancer: a review of adjunct therapies including paricalcitol, hydroxychloroquine, intravenous vitamin C, statins, metformin, curcumin, and aspirin. Cancer Manag Res 2018;10:2003-18. [Crossref] [PubMed]
- Lawrence JA, Akman SA, Melin SA, et al. Oral paricalcitol (19-nor-1,25-dihydroxyvitamin D2) in women receiving chemotherapy for metastatic breast cancer: a feasibility trial. Cancer Biol Ther 2013;14:476-80. [Crossref] [PubMed]
- Gombart AF, Luong QT, Koeffler HP. Vitamin D compounds: activity against microbes and cancer. Anticancer Res 2006;26:2531-42. [PubMed]
- Schwartz GG, Eads D, Naczki C, et al. 19-nor-1 alpha,25-dihydroxyvitamin D2 (paricalcitol) inhibits the proliferation of human pancreatic cancer cells in vitro and in vivo. Cancer Biol Ther 2008;7:430-6. [Crossref] [PubMed]
- Mullin GE, Dobs A. Vitamin d and its role in cancer and immunity: a prescription for sunlight. Nutr Clin Pract 2007;22:305-22. [Crossref] [PubMed]
(English Language Editor: J. Jones)









