
The effect of bone cement on the curative effect of percutaneous kyphoplasty in the treatment of osteoporotic vertebral compression fracture
Introduction
Osteoporotic vertebral compression fracture (OVCF) is a common fracture that is mainly characterized by severe lower back pain and movement limitation (1). With population aging, the incidence of osteoporosis, which causes more than 8.9 million fractures each year globally, is gradually increasing. The average lifetime risk of fracture to the wrist, hip, or spine is 40%, which is equal to the risk of developing cardiovascular disease (2). Among these fractures, osteoporotic vertebral fractures (OVFs) carry the highest mortality rate, which is even higher than that of hip fractures (3). Approximately 1.4 million patients receive clinical treatment for vertebral compression fractures globally each year, and most patients can obtain satisfactory efficacy through conservative treatment or surgery. However, conservative treatment requires prolonged immobilization in bed, which can aggravate osteoporosis and increase the incidence of complications and the risk of death (4-6).
Percutaneous kyphoplasty (PKP) has been widely used in the treatment of OVCF. This method involves expanding the compressed vertebra through a balloon to reposition the vertebral body and creating a cavity in the vertebral body into which bone cement is injected, thereby enhancing the strength of the vertebral body and restoring spinal stability (7). PKP surgery has the characteristics of less trauma and relieving pain (8,9), as well as the advantages of correcting the Cobb angle and the height of the vertebral body (10), reducing the occurrence of complications, and shortening the hospitalization time (11).
Bone cement is a commonly used perfusion agent in PKP surgery. Differences in the distribution of bone cement in the vertebral body affect its biomechanical properties, thereby influencing the therapeutic effect (12). However, in current clinical treatment, there is no fixed standard for the optimal distribution and viscosity of bone cement in the vertebral body in patients with OVCF. Therefore, in the present study, we selected patients with OVCF who received PKP in our hospital to explore the effect of bone cement viscosity and distribution in the vertebral body on the curative effect in these patients, with the aim of providing a reference for the effective clinical use of PKP in OVCF treatment. We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/apm-21-2767).
Methods
Clinical cases
A total of 323 patients who underwent PKP to treat OVCF in the First Affiliated Hospital of Chongqing Medical University between January 2018 and June 2020 were enrolled. According to the viscosity of the bone cement, the patients were divided into a high viscosity group (HV group, n=161) and a low viscosity group (LV group, n=162). Based on the lateralization of the bone cement in the vertebral body, the patients were also divided into a central group (n=169) and a lateral group (n=154).
The criteria for patients’ inclusion in the study were as follows: (I) determined to have osteoporosis following bone mineral density (BMD) examination (13); (II) aged ≥65 years old; (III) accompanied with acute vertebral fracture-related back pain; (IV) degree of vertebral compression <75%; (V) had not received PKP previously; (VI) with complete imaging examination data.
The exclusion criteria were as follows: (I) patients with traumatic fractures; (II) patients with pathological vertebral compression fractures caused by bone tumors; (III) patients with secondary osteoporosis; (IV) patients with infectious diseases, such as tuberculosis, or who had undergone vertebroplasty, chest, or lumbar spine surgery; (V) patients with lung, liver, and kidney dysfunction or autoimmune diseases. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University, and all patients signed an informed consent form.
Surgical methods
The operation was performed following the methods described by Li et al. (14). Bucinnazine was routinely injected 30 minutes before surgery, and electrocardiogram monitoring was conducted throughout the operation. The bone cement used for the procedure was precooled for 24 hours. With the patient lying prone and the abdomen suspended, the vertebral body was repositioned by external compression under fluoroscopic observation. A digital silhouette angiography (DSA) X-ray machine was used to guide and locate the injured vertebrae and pedicle projection points (unilateral pedicle approach was used for T10 and above, and bilateral pedicle approach was used for below T10). Local anesthesia was performed with lidocaine 1%, and the puncture needle was pulled inwardly into the vertebral body through the pedicle, with the front position of the fluoroscope reaching the shadow of the pedicle inner wall and the lateral position crossing the vertebral body posterior edge. The inner core was then pulled out, the guide needle was inserted, and the working cannula was settled along with the guide pin. After pathological tissue had been obtained with conventional biopsy, under the continuous guidance of DSA, a fine drill was used to drill slowly to the junction of one-third of the anterior and middle parts of the vertebral body through the working cannula. After the fine drill had been taken out, a balloon was put in for expansion, which was maintained for 1 minute under the support of the balloon. Finally, the bone cement was stirred and an appropriate amount was injected inside the cavity in the vertebral body after the removal of the balloon during the drawing period.
During the operation, attention was paid to the following: (I) the entire operation was performed under DSA fluoroscopy. (II) To ensure the dispersion of the bone cement in the vertebral body, a small amount of high-viscosity bone cement was injected into the vertebral body in the early stage of solidification. Later, under fluoroscopy, the bone cement was injected until it dispersed into the anterior and middle columns of the vertebral body. (III) If bone cement leakage occurred during the operation, the injection was stopped immediately. (IV) After injection of the bone cement, the working cannula was first rotated and then removed to prevent the bone cement from leaking. (V) For single-pass vertebral bodies, it was ensured that the cement passed through the midline of the vertebral body to avoid leakage of bone cement, and the amount of bone cement injected into the vertebral body was 3–5 mL; for double-pass vertebral bodies, 5–10 mL cement was used. After the operation, the patient was kept on the bed for 24 hours, with only turning over permitted. On the second day, the anti-osteoporotic treatment was performed.
Observation indexes
Clinical baseline characteristics of the patients were collected including age, sex, body mass index (BMI), bone density, fracture level (lower thorax, mid thorax, or lumbar spine), fracture type (wedge, double concave, or crush), severity (mild, moderate, or severe), and follow-up time. Relevant perioperative parameters including operative time, blood loss, hospitalization time, bone cement injection volume, bone cement viscosity (HV or LV), and bone cement distribution (central or lateral) were also recorded. To evaluate the curative effect, the visual analog scale (VAS) score of the posterior back, Oswestry dysfunction index (ODI), Cobb angle, vertebral body height, anterior vertebral height (AVH) of the fractured vertebral body, and complications were recorded before the operation, and at 24 hours and 3 months after the operation.
Statistical analysis
Data were analyzed using SPSS10.0 software (IBM Corp., Armonk, NY, USA). The t-test was used for comparisons between two groups, and one-way analysis of variance was used for comparisons between multiple groups. The results were expressed as mean ± standard deviation (SD), with P<0.05 representing statistical significance.
Results
Clinical baseline characteristics
The clinical characteristics of the study participants were recorded. There were no statistical differences between the HV group and the LV group or between the central group and the lateral group in terms of age, sex, BMI, bone density, fracture level, fracture type, severity, and follow-up time (P>0.05) (Tables 1,2).
Table 1
| Items | LV group (n=162) | HV group (n=161) | t/χ2 | P |
|---|---|---|---|---|
| Age (years) | 67.59±3.97 | 68.07±4.38 | −1.036 | 0.301 |
| Sex | 0.004Ä | 0.950 | ||
| Male | 72 | 71 | ||
| Female | 90 | 90 | ||
| BMI | 28.75±0.85 | 28.84±1.06 | −0.815 | 0.416 |
| Bone density | −2.72±0.50 | −2.81±0.45 | −1.536 | 0.126 |
| Fracture level | 0.051Ä | 0.975 | ||
| Lower thorax | 43 | 41 | ||
| Mid thorax | 37 | 37 | ||
| Lumbar spine | 82 | 83 | ||
| Fracture type | 0.007Ä | 0.997 | ||
| Wedge | 57 | 57 | ||
| Double concave | 52 | 51 | ||
| Crush | 53 | 53 | ||
| Severity | 0.532Ä | 0.766 | ||
| Mild | 34 | 35 | ||
| Moderate | 21 | 25 | ||
| Severe | 107 | 101 | ||
| Follow-up (months) | 17.94±3.89 | 18.33±3.65 | −0.931 | 0.352 |
Ä, means the data were analyzed by χ2. LV, low viscosity; HV, high viscosity; BMI, body mass index.
Table 2
| Items | Central group (n=169) | Lateral group (n=154) | t/χ2 | P |
|---|---|---|---|---|
| Age (years) | 67.66±3.48 | 68.02±4.84 | −0.754 | 0.451 |
| Sex | 1.921Ä | 0.166 | ||
| Male | 81 | 62 | ||
| Female | 88 | 92 | ||
| BMI | 28.75±0.86 | 28.84±1.06 | −0.879 | 0.380 |
| Bone density | −2.77±0.48 | −2.76±0.47 | 0.026 | 0.979 |
| Fracture level | 0.759Ä | 0.684 | ||
| Lower thorax | 43 | 41 | ||
| Mid thorax | 42 | 32 | ||
| Lumbar spine | 84 | 81 | ||
| Fracture type | 2.282Ä | 0.320 | ||
| Wedge | 54 | 60 | ||
| Double concave | 54 | 49 | ||
| Crush | 61 | 45 | ||
| Severity | 3.528Ä | 0.171 | ||
| Mild | 42 | 27 | ||
| Moderate | 20 | 26 | ||
| Severe | 107 | 101 | ||
| Follow-up (months) | 18.26±3.84 | 17.99±3.70 | 0.635 | 0.526 |
Ä, means the data were analyzed by χ2. BMI, body mass index.
Comparison of perioperative parameters between the groups of patients
Relevant perioperative parameters were compared between the groups of patients. The results showed that in the HV group, the operative time was markedly shorter and the blood loss volume and hospitalization time were slightly reduced compared to those in the LV group. Also, the bone cement injection volume was slightly higher in the HV than the LV group. In the HV group, patients with centrally distributed bone cement significantly outnumbered those with laterally distributed bone cement (P<0.05) (Table 3). When the operative time, blood loss volume, hospitalization time, and bone cement volume were compared between the central group and the lateral group, no significant differences were found. However, the viscosity of the bone cement in the central group was significantly higher than that in the lateral group (P<0.05) (Table 4).
Table 3
| Items | LV group (n=162) | HV group (n=161) | t/χ2 | P |
|---|---|---|---|---|
| Operation time (min) | 28.26±4.52 | 23.40±3.50 | 10.798 | 0.000* |
| Blood loss (mL) | 16.87±2.02 | 16.56±1.72 | 1.490 | 0.137 |
| Hospitalization time (days) | 4.67±1.69 | 4.32±1.47 | 1.914 | 0.056 |
| Bone cement injection volume (mL) | 4.20±0.55 | 4.24±0.57 | −0.497 | 0.620 |
| Bone cement distribution | 8.085Ä | 0.004* | ||
| Central | 72 | 97 | ||
| Lateral | 90 | 64 |
Ä, means the data were analyzed by χ2; *, P<0.05. LV, low viscosity; HV, high viscosity.
Table 4
| Items | Central group (n=169) | Lateral group (n=154) | t/χ2 | P |
|---|---|---|---|---|
| Operation time (min) | 25.36±4.23 | 26.37±5.16 | −1.929 | 0.055 |
| Blood loss (mL) | 16.73±1.92 | 16.69±1.84 | 0.185 | 0.853 |
| Hospitalization time (days) | 4.47±1.56 | 4.53±1.63 | −0.296 | 0.767 |
| Bone cement injection volume (mL) | 4.21±0.57 | 4.23±0.55 | −0.287 | 0.774 |
| Bone cement viscosity | 8.085Ä | 0.004* | ||
| HV | 72 | 90 | ||
| LV | 97 | 64 |
Ä, means the data were analyzed by χ2; *, P<0.05. HV, high viscosity; LV, low viscosity.
High-viscosity and centrally distributed bone cement is related to better recovery in patients with OVCF
To determine the effects of bone cement viscosity and distribution in the treatment of patients with OVCF, we next conducted VAS and ODI score evaluations. No obvious difference was observed in the VAS or ODI scores of the HV and LV groups before the operation, at 3 months after the operation, or at the final follow-up (P>0.05). However, at 24 hours after surgery, the ODI score in the HV group was markedly lower than that in the LV group (P<0.05), but there was no difference in the VAS score (P>0.05) (Figure 1A,1B). Furthermore, at 24 hours after the operation, the VAS score in the lateral group was notably higher than that in the central group (P<0.05). At the final follow-up, the ODI in the lateral group was markedly higher than that in the central group (P<0.05). During follow-up, there was no obvious difference in the VAS or ODI score between the central and lateral groups (P>0.05) (Figure 1C,1D). These results indicated that high-viscosity and centrally distributed bone cement reduces the incidence of pain and dysfunction, and thus, is more conducive to the recovery of patients with OVCF after surgery.
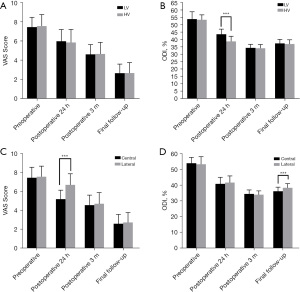
High-viscosity and centrally distributed bone cement can better correct the vertebral body in patients with OVCF
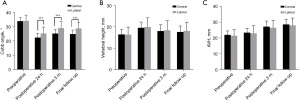
To further directly evaluate the effects of bone cement distribution and viscosity on the treatment effect and recovery of patients with OVCF, we performed imaging examinations of the vertebral bodies of patients treated with high-viscosity and centrally distributed bone cement (Figures 2,3). As shown in Figure 4A-4C, before surgery, there was no distinct difference in the Cobb angle, vertebral height, or AVH between the HV group and the LV group (P>0.05), which indicated the disease severity to be similar between the two groups. Compared with that in the LV group, the Cobb angle in the HV group was obviously reduced at 24 hours after surgery (P<0.05), and at both the 3-month and the final follow-up, the AVH in the HV group was markedly increased (P<0.05).
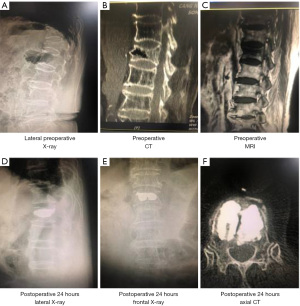
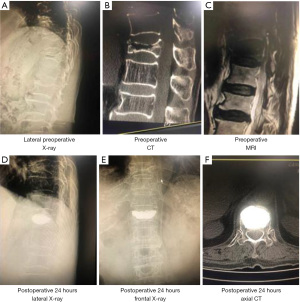
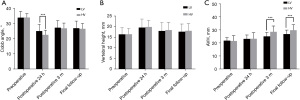
Before surgery, there was no obvious difference in the Cobb angle, vertebral height, or AVH between the central and lateral groups (P>0.05). However, the Cobb angle in the lateral group was significantly larger than that in the central group at 24 hours after surgery, 3 months after surgery, and at the last follow-up (P<0.05), although the differences in vertebral body height and AVH were not significant (P>0.05) (Figure 5A-5C). These results indicated that high-viscosity and centrally distributed bone cement shows better efficacy in the treatment of OVCF, with a lower possibility of recurrence.
High-viscosity and centrally distributed bone cement reduces postoperative complications
We further investigated the occurrence of complications after treatment with bone cement of differing viscosity and distribution. Vertebral body refracture, vertebral body recompression, wound infection, and nerve injury displayed no differences in incidence between the HV group and the LV group. However, the incidence of bone cement leakage in the HV group was markedly lower than that in the LV group (P<0.05) (Table 5). Further, the incidences of vertebral body recompression, wound infection, and nerve injury did not differ significantly between the lateral group and the central group, although the incidences of bone cement leakage and vertebral body refracture in the central group were dramatically lower than those in the lateral group (P<0.05) (Table 6).
Table 5
| Items | LV group (n=162), n (%) | HV group (n=161), n (%) |
|---|---|---|
| Bone cement leakage | 33 (20.37) | 18 (11.19)* |
| Vertebral body refracture | 24 (14.81) | 27 (16.77) |
| Vertebral body recompression | 20 (12.35) | 20 (12.42) |
| Wound infection | 11 (6.79) | 11 (6.83) |
| Nerve injury | 12 (7.41) | 11 (6.83) |
*, P<0.05. LV, low viscosity; HV, high viscosity.
Table 6
| Items | Central group (n=169), n (%) | Lateral group (n=154), n (%) |
|---|---|---|
| Bone cement leakage | 17 (10.06)* | 34 (22.08) |
| Vertebral body refracture | 17 (10.06)* | 35 (22.73) |
| Vertebral body recompression | 20 (11.83) | 20 (12.99) |
| Wound infection | 10 (5.91) | 13 (8.44) |
| Nerve injury | 12 (7.10) | 12 (7.79) |
*, P<0.05.
Discussion
Osteoporosis is a systemic bone disease that results in bone mass reduction and bone microarchitecture degradation. It is especially common among elderly people over the age of 60 years in China, which has a high incidence of osteoporosis, is home to about 8.2 million patients with the condition (15,16). OVCF is a common complication of osteoporosis, and vertebral fissures or anterior vertebral collapse can cause fractures (17). Population aging has seen a rise in the incidence of OVCF, which can lead to spinal deformity. In some severe cases, patients are faced with unbearable pain and are even unable to take care of themselves, which seriously reduces their life quality while increasing their economic burden (18).
The treatment aims for patients with OVCF are to restore mobility, reduce pain, and avoid new fractures. Conventional conservative treatments include bed rest, opioid analgesia, and external fixation support to relieve pain and strengthen the spine (19). However, long-term bed rest can cause a variety of complications, such as pneumonia, urinary tract infections, bedsores, and deep vein thrombosis, and conservative treatments can aggravate bone demineralization, thus increasing the risk of fractures (20). At present, PKP is commonly used in the clinical treatment of OVCF and involves reconstructing the injured vertebral body by injecting bone cement. Bone cement can be distributed throughout the whole vertebral body and after solidification, can stabilize and support the injured vertebral body well. PKP is widely used in the clinical setting because it entails minimal trauma, is easy to perform, has good efficacy, and allows for quick recovery (8,21). However, studies have shown that the biomechanical properties of the bone cement injected into the vertebral body can be affected by its distribution and viscosity, which in turn impacts the therapeutic effect (12). Liu et al. reported that high-viscosity bone cement can reduce the risk of puncture and leakage (22). Zou also reported that increasing the viscosity of bone cement from low to high reduced the cement leakage rate from 40% to 2%. These data confirm the superiority of high-viscosity over low-viscosity bone cement in the treatment of OVCF (23). Furthermore, reducing the bone cement viscosity has been shown to increase the distance of bone cement diffusion, thus increasing the incidence of refracture and reducing the surgical efficacy (24).
In this study, compared with the LV group, the HV group had a significantly shorter operative time, a reduced ODI score and Cobb angle at 24 hours postoperatively, significantly increased AVH at the 3-month and final follow-ups, and a lower incidence of bone cement leakage. Compared to the lateral group, the central group had a significantly reduced VAS score and Cobb angle at 24 hours postoperatively, and showed dramatic reductions in the Cobb angle and in the incidences of bone cement leakage and vertebral body refracture at the 3-month and final follow-ups. These results suggest that high-viscosity and centrally distributed bone cement may reduce the risk for patients with spinal dysfunction and aggravation of kyphosis, and have an analgesic effect, thus making it more effective in the treatment of OVCF.
Conclusions
In summary, the distribution and viscosity of bone cement affects the clinical efficacy of PKP surgery in patients with OVCF. High-viscosity bone cement can reduce the risk of bone cement leakage and vertebral refracture, while central distribution of the bone cement has a pain-relieving effect. Therefore, high-viscosity bone cement with central distribution should be used in PKP in patients with OVCF to promote better treatment outcomes.
Acknowledgments
Funding: Technological Innovation and Application Development Projects of Chongqing (cstc2020jscx-msxmX0062).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/apm-21-2767
Data Sharing Statement: Available at https://dx.doi.org/10.21037/apm-21-2767
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apm-21-2767). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University, and all patients signed an informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cooper C, Atkinson EJ, O'Fallon WM, et al. Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985-1989. J Bone Miner Res 1992;7:221-7. [Crossref] [PubMed]
- Sanli I, van Kuijk SMJ, de Bie RA, et al. Percutaneous cement augmentation in the treatment of osteoporotic vertebral fractures (OVFs) in the elderly: a systematic review. Eur Spine J 2020;29:1553-72. [Crossref] [PubMed]
- Cauley JA, Thompson DE, Ensrud KC, et al. Risk of mortality following clinical fractures. Osteoporos Int 2000;11:556-61. [Crossref] [PubMed]
- Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006;17:1726-33. [Crossref] [PubMed]
- Pukenas BA, Jensen ME. Percutaneous vertebral augmentation. In: Hurst RW, Rosenwasser RH. editors. Neurointerventional management: diagnosis and treatment. 2nd ed. Boca Raton: CRC Press, 2012:570-97.
- Gerling MC, Eubanks JD, Patel R, et al. Cement augmentation of refractory osteoporotic vertebral compression fractures: survivorship analysis. Spine (Phila Pa 1976) 2011;36:E1266-9. [Crossref] [PubMed]
- Abduljabbar FH, Al-Jurayyan A, Alqahtani S, et al. Does balloon kyphoplasty deliver more cement safely into osteoporotic vertebrae with compression fractures compared with vertebroplasty? A study in vertebral analogues. Global Spine J 2015;5:300-7. [Crossref] [PubMed]
- Zhang Y, Shi L, Tang P, et al. Comparison of the efficacy between two micro-operative therapies of old patients with osteoporotic vertebral compression fracture: a network meta-analysis. J Cell Biochem 2017;118:3205-12. [Crossref] [PubMed]
- He X, Li H, Meng Y, et al. Percutaneous kyphoplasty evaluated by cement volume and distribution: an analysis of clinical data. Pain Physician 2016;19:495-506. [PubMed]
- Wang F, Wang LF, Miao DC, et al. Which one is more effective for the treatment of very severe osteoporotic vertebral compression fractures: PVP or PKP? J Pain Res 2018;11:2625-31. [Crossref] [PubMed]
- Chen B, Fan S, Zhao F. Percutaneous balloon kyphoplasty of osteoporotic vertebral compression fractures with intravertebral cleft. Indian J Orthop 2014;48:53-9. [Crossref] [PubMed]
- Xu XK, Zeng WW, Zhou YJ. The relationship between the distribution of bone cement in OVCFs vertebral body and its biomechanical properties. Shenzhen Journal of Integrated Traditional Chinese and Western Medicine 2018;28:11-3.
- Nakamura T. WHO diagnostic criteria for osteoporosis and trends in Europe and USA. Nihon Rinsho 2004;62:235-9. [PubMed]
- Li K, Ji C, Luo D, et al. Role of percutaneous vertebroplasty with high-viscosity cement in the treatment of severe osteoporotic vertebral compression fractures. Sci Rep 2021;11:4602. [Crossref] [PubMed]
- Zeng TH, Wang YM, Yang XJ, et al. The clinical comparative study on high and low viscosity bone cement application in vertebroplasty. Int J Clin Exp Med 2015;8:18855-60. [PubMed]
- Osteoporosis and Bone Mineral Disease Branch of Chinese Medical Association. Epidemiological survey of osteoporosis in China and release of the results of the "Healthy Bone" campaign. Chinese Journal of Osteoporosis and Bone Mineral Research 2019;12:317-8.
- Kan SL, Yuan ZF, Chen LX, et al. Which is best for osteoporotic vertebral compression fractures: balloon kyphoplasty, percutaneous vertebroplasty or non-surgical treatment? A study protocol for a Bayesian network meta-analysis. BMJ Open 2017;7:e012937 [Crossref] [PubMed]
- Li J, Jiang Y. Clinical diagnosis and treatment of osteoporotic vertebral compression fractures. Chinese Journal for Clinicians 2018;46:1389-92.
- Zhao YM, Jing PJ. Non-surgical management of osteoporotic vertebra compression fractures. West China Medical Journal 2019;34:1063-7.
- Zhang H, Xu C, Zhang T, et al. Does percutaneous vertebroplasty or balloon kyphoplasty for osteoporotic vertebral compression fractures increase the incidence of new vertebral fractures? A meta-analysis. Pain Physician 2017;20:E13-28. [Crossref] [PubMed]
- Cao LY, Jiang MJ, Lin BZ, et al. Recovery of vertebral body height and related influencing factors in the treatment of osteoporotic vertebral compression fracture with high viscosity bone cement vertebroplasty. Chinese and Foreign Medical Research 2020;18:48-50.
- Liu T, Li Z, Su Q, et al. Cement leakage in osteoporotic vertebral compression fractures with cortical defect using high-viscosity bone cement during unilateral percutaneous kyphoplasty surgery. Medicine (Baltimore) 2017;96:e7216 [Crossref] [PubMed]
- Zou XN. Vertebral height restoration and its associative factors during high-viscosity bone cement vertebroplasty in the treatment of osteopomsis vertebral compression fractures. Chinese Journal of Spine and Spinal Cord 2017;27:991-6.
- Tang S, Fu W, Zhang H, et al. Efficacy and safety of high-viscosity bone cement vertebroplasty in treatment of osteoporotic vertebral compression fractures with intravertebral cleft. World Neurosurg 2019;132:e739-45. [Crossref] [PubMed]
(English Language Editor: J. Reynolds)


