Association between blood urea nitrogen and 30-day mortality in patients with sepsis: a retrospective analysis
Introduction
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection. Severe sepsis and septic shock affect millions of people around the world each year, and more than a quarter of these patients die (1). The mortality rate of septic patients hospitalized in intensive care unit (ICU) is as high as 41.9% (2). Death-risk stratification in septic patients enables early identification of patients at high risk of death and facilitates rational allocation of medical resources to improve outcomes. Scoring tools such as Acute Physiology and Chronic Health Evaluation (APACHE) can be used for risk stratification in septic patients, but its implementation is relatively cumbersome and can be easily influenced by some highly variable parameters such as heart rate and respiration (3). Therefore, there is a need to find clinically common and practicable markers for the rapid death-risk stratification of septic patients, which is also an important research direction of the Surviving Sepsis Campaign (4).
Blood urea nitrogen (BUN) is the main end product of protein metabolism in the human body and is excreted mainly by the kidneys. BUN level will increase when there is excessive protein breakdown or when the glomerular filtration rate decreases. Thus, BUN level can reflect protein catabolism in the human body (5) and is also a marker of renal impairment. The rate of protein catabolism increases significantly in patients with sepsis (6), and sepsis is often complicated with acute renal injury (7). These factors can lead to an increase in BUN levels in patients with sepsis. Meanwhile, the BUN test is simple and common in clinical laboratories.
Previous studies have suggested that elevated BUN level may independently predict mortality in critically ill patients (8,9); however, these studies had small sample sizes, and a large-sample study of 30-day mortality risk stratification using BUN level in patients with sepsis has yet to be performed. By using the Medical Information Mart for Intensive Care III (MIMIC-III) database, we performed this real-world study with a large sample size to investigate the relationship between BUN level and 30-day mortality in patients with sepsis. We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/apm-21-2937).
Methods
Data source
The data of this retrospective cohort study were sourced from the MIMIC-III database (available at https://mimic.mit.edu/), which collects detailed information on the routine clinical care of more than 60,000 patients admitted to the ICU at Beth Israel Deaconess Medical Center (BIDMC) in Boston, Massachusetts, USA. The MIMIC-III data set is freely available to researchers around the world and has been widely used in the development of predictive models, epidemiological studies, and educational courses (10). MIMIC-III (v.1.4) contains data associated with 38,597 adult patients admitted to critical care units between 2001 and 2012 (10). All patient data were divided into different lists and organized into tables in the corresponding CSV format, including (in total) 26 tables, which are available for interested researchers to query and download (11). MIMIC-III is connected to the Social Security Database, which records other patient information, such as follow-up time and outcomes. The information related to personal privacy in the database is processed to protect privacy; for example, all the patients are anonymous and are referred to by an individual ID. The MIMIC data set does not provide a real date of admission, data of birth, or date of death; rather, it uses a mathematical model that randomly adds or subtracts the same values for the above variables. An author of this study completed a series of courses offered by the National Institutes of Health, passed the examinations, and was thus authorized to use the relevant information from the MIMIC-III database (certification No. 40764077).
Research population
The inclusion criteria were as follows: (I) all patients admitted to the ICU in the MIMIC-III database from June 2001 to October 2012, and (II) patients meeting the diagnostic criteria of sepsis according to the new criteria for sepsis (Sepsis-3); that is, an increase of 2 points or more on the sequential organ failure assessment (SOFA) in patients with suspected infection (12). The exclusion criteria were the following: (I) patients who were not admitted to the ICU for the first time, (II) patients younger than 18 years, (III) patients with incomplete BUN measurement within the first day of ICU admission or with extremely abnormal BUN levels on the first measurement, and (IV) patients with an inaccurate time of death recorded. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The MIMIC database has been approved by the Institutional Review Board (IRB) of MIT and BIDMC, both of which waived informed consent for studies related to the MIMIC-III database; therefore, our current study did not need an approval from the ethics committee of our hospital.
Variables and data
Baseline variables extracted from the MIMIC-III database included demographic features (gender, age, body mass index), type of ICU, vital signs, comorbidities, life-support measures, and laboratory test results (within the first day after admission). The type of ICU included medical ICU (MICU), surgical/trauma surgical ICU (SICU/TSICU), and coronary care unit (CCU)/cardiac surgery recovery unit (CSRU). Vital signs included heart rate, mean arterial pressure (MAP), respiratory rate, body temperature, and oxygen saturation. The comorbidities included congestive heart failure (CHF), arrhythmia, hypertension, stroke, chronic obstructive pulmonary disease (COPD), diabetes mellitus (DM), chronic renal failure, liver disease, malignancy, and coagulation disorders. Life support measures within 24 hours after admission included mechanical ventilation (MV), renal replacement therapy (RRT), and use of hypertensors. Laboratory tests included the measurements of blood white blood cell (WBC) count, hemoglobin, platelet count, blood potassium, and blood sodium.
Data were queried and extracted using Structured Query Language (SQL) with the open-source PostgreSQL (v9.6) software and its GUI software, Navicat [v.12.1.11(64-bit)-Premium].
The objective of this study was to explore the relationship between BUN level and 30-day mortality in patients with sepsis.
Statistical analysis
Normally distributed baseline measurement data are expressed as mean ± standard deviation (), and the nonnormally distributed data are presented as medians and interquartile range. Count data are expressed as frequencies with percentages. Multiple interpolation was applied for missing data in very few cases. For the analysis of baseline features, statistical differences in continuous variables among five BUN groups were analyzed using one-way analysis of variance (ANOVA), and categorical variables were analyzed using chi-square test. Hazard ratios (HRs) and 95% confidence intervals (CIs) for deaths in different BUN groups were calculated using multivariate Cox regression analysis. All the covariates in Table 1 were included in the regression model for adjustment to control for confounding effects. A generalized additive model was used to identify the nonlinear relationship between BUN level and 30-day mortality. A two-segment linear regression model was created based on the smoothed curves. A segmented relationship is defined by the slope parameters and the break-points where the linear relation changes, and R package “Segmented” was used (13). Comparison of the two-segment linear regression model and the simple linear model was performed using the log-likelihood ratio test.
Table 1
| Variables | Total | BUN quintiles (mg/dL) | P value | ||||
|---|---|---|---|---|---|---|---|
| Q1 [≤12] | Q2 [13–18] | Q3 [19–27] | Q4 [28–43] | Q5 [≥44] | |||
| Participants | 12,713 | 2,160 | 2,529 | 2,833 | 2,538 | 2,653 | |
| Gender, n (%) | <0.001 | ||||||
| Female | 5,998 (47.2) | 1,210 (56.0) | 1,169 (46.2) | 1,320 (46.6) | 1,188 (46.8) | 1,111 (41.9) | |
| Male | 6,715 (52.8) | 950 (44.0) | 1,360 (53.8) | 1,513 (53.4) | 1,350 (53.2) | 1,542 (58.1) | |
| Age (years), mean ± SD | 67.2±16.5 | 56.7±17.7 | 65.4±16.5 | 69.8±15.1 | 71.3±14.7 | 70.6±14.5 | <0.001 |
| Weight (kg), mean ± SD | 80.9±25.3 | 77.9±25.7 | 81.4±24.9 | 80.7±24.1 | 80.4±24.9 | 83.7±26.7 | <0.001 |
| Service unit, n (%) | <0.001 | ||||||
| MICU | 6,345 (49.9) | 932 (43.1) | 1,015 (40.1) | 1,350 (47.7) | 1,386 (54.6) | 1,662 (62.6) | |
| SICU/TSICU | 3,303 (26.0) | 783 (36.2) | 835 (33.0) | 736 (26.0) | 533 (21.0) | 416 (15.7) | |
| CCU/CSRU | 3,065 (24.1) | 445 (20.6) | 679 (26.8) | 747 (26.4) | 619 (24.4) | 575 (21.7) | |
| Vital signs, mean ± SD | |||||||
| Heart rate (bpm) | 88.3±16.6 | 91.2±16.0 | 88.4±15.8 | 87.7±16.4 | 87.9±17.1 | 86.6±17.1 | <0.001 |
| MAP (mmHg) | 76.4±10.8 | 79.2±10.6 | 77.9±10.1 | 76.6±10.4 | 75.2±10.6 | 73.9±11.4 | <0.001 |
| Respiratory rate (bpm) | 19.9±4.4 | 19.4±4.3 | 19.5±4.3 | 19.9±4.4 | 20.1±4.4 | 20.3±4.4 | <0.001 |
| Temperature (°C) | 36.9±0.7 | 37.1±0.7 | 37.0±0.7 | 36.9±0.7 | 36.8±0.7 | 36.7±0.7 | <0.001 |
| SpO2 (%) | 96.9±2.8 | 97.4±1.9 | 97.1±2.5 | 96.9±3.1 | 96.7±3.1 | 96.7±3.0 | <0.001 |
| Comorbidities, n (%) | |||||||
| CHF | 2,893 (22.8) | 221 (10.2) | 405 (16.0) | 649 (22.9) | 746 (29.4) | 872 (32.9) | <0.001 |
| Cardiac arrhythmias | 3,064 (24.1) | 325 (15.0) | 493 (19.5) | 694 (24.5) | 752 (29.6) | 800 (30.2) | <0.001 |
| Hypertension | 1,738 (13.7) | 40 (1.9) | 111 (4.4) | 289 (10.2) | 482 (19.0) | 816 (30.8) | <0.001 |
| Stroke | 459 (3.6) | 100 (4.6) | 117 (4.6) | 104 (3.7) | 70 (2.8) | 68 (2.6) | <0.001 |
| COPD | 2,665 (21.0) | 389 (18.0) | 525 (20.8) | 640 (22.6) | 559 (22.0) | 552 (20.8) | 0.001 |
| DM | 3,535 (27.8) | 317 (14.7) | 551 (21.8) | 739 (26.1) | 909 (35.8) | 1,019 (38.4) | <0.001 |
| Renal failure | 2,132 (16.8) | 47 (2.2) | 115 (4.5) | 352 (12.4) | 603 (23.8) | 1,015 (38.3) | <0.001 |
| Liver disease | 1,044 (8.2) | 198 (9.2) | 178 (7.0) | 193 (6.8) | 205 (8.1) | 270 (10.2) | <0.001 |
| Malignancy | 1,336 (10.5) | 221 (10.2) | 258 (10.2) | 302 (10.7) | 287 (11.3) | 268 (10.1) | 0.611 |
| Coagulopathy | 2,277 (17.9) | 316 (14.6) | 357 (14.1) | 463 (16.3) | 498 (19.6) | 643 (24.2) | <0.001 |
| LST, n (%) | |||||||
| MV use (1st 24 h) | 6,397 (50.3) | 1,192 (55.2) | 1,388 (54.9) | 1,465 (51.7) | 1,192 (47.0) | 1,160 (43.7) | <0.001 |
| RRT use (1st 24 h) | 638 (5.0) | 22 (1.0) | 28 (1.1) | 73 (2.6) | 155 (6.1) | 360 (13.6) | <0.001 |
| Vasopressor use (1st 24 h) | 2,199 (17.3) | 352 (16.3) | 415 (16.5) | 454 (16.1) | 451 (17.8) | 527 (19.9) | <0.001 |
| Laboratory tests | |||||||
| WBC (×109/L), median (IQR) | 11.4 (7.9, 16.2) | 10.7 (7.4, 15.4) | 11.0 (7.8, 15.1) | 11.4 (8.1, 16.2) | 11.8 (8.0, 17.0) | 12.1 (8.2, 17.5) | <0.001 |
| Hemoglobin (g/dL), mean ± SD | 10.6±2.0 | 10.8±2.0 | 10.9±2.0 | 10.7±2.0 | 10.5±1.9 | 10.1±1.8 | <0.001 |
| Platelets (×109/L), median (IQR) | 197.0 (134.0, 274.0) | 202.0 (133.0, 284.0) | 194.0 (134.0, 267.0) | 198.0 (138.0, 274.0) | 197.0 (133.0, 273.0) | 195.0 (128.0, 274.0) | 0.02 |
| Potassium (mmol/L), mean ± SD | 4.2±0.8 | 3.9±0.7 | 4.0±0.7 | 4.1±0.7 | 4.2±0.8 | 4.5±0.9 | <0.001 |
| Sodium (mmol/L), mean ± SD | 138.7±5.5 | 138.4±4.7 | 138.5±4.8 | 138.8±4.8 | 139.0±5.5 | 138.9±7.1 | <0.001 |
BUN, blood urea nitrogen; MICU, medical intensive care unit; SICU, surgical intensive care unit; TSICU, trauma surgical intensive care unit; CCU, coronary care unit; CSRU, cardiac surgery recovery unit; MAP, mean arterial pressure; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; LST, life support treatment; MV, mechanical ventilation; RRT, renal replacement therapy; WBC, white blood cell.
Statistical analyses were performed using R Statistical Software (https://www.r-project.org, The R Foundation) and Free Statistics analysis platform. All P values reported are two-tailed, and values of P<0.05 were considered to be statistically significant.
Results
Study population and baseline features
A total of 20,318 septic patients admitted to the ICU were screened from the MIMIC-III database. After 5,278 patients with multiple ICU admissions were excluded, the remaining 15,040 patients who were admitted to the ICU for the first time were further screened. After 223 patients who were younger than 18 years, 2,041 patients with incomplete or extremely abnormal BUN records within the first day after ICU admission, and 63 patients with inaccurate time of death recorded were excluded, 12,713 patients were finally included in this retrospective cohort study (Figure 1). All patients completed 30-day follow-up.

The total population was divided equally into 5 groups based on the BUN level. Since all BUN measurements in the original records were integers, Q1 was defined as BUN ≤12 mg/dL, Q2 as BUN 13–18 mg/dL, Q3 as 19–27 mg/dL, Q4 as 28–43 mg/dL, and Q5 as ≥44 mg/dL. Demographic features (gender, age, body mass index), type of ICU admission, vital signs, comorbidities, life-support measures, and laboratory test results are presented as a total of all participants and by group (Table 1).
Sensitivity analysis for the relationship between BUN level and 30-day mortality in patients with sepsis
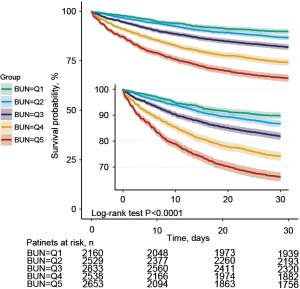
Up to 2,623 of the 12,713 patients died within 30 days after ICU admission, yielding a mortality rate of 20.6%. By calculating the HR and 95% CI of BUN level to 30-day mortality, we found in three models (without adjustment, moderate adjustment, and with all covariates included in the adjustment) that the risk of death gradually increased in groups Q2, Q3, Q4, and Q5 with increasing BUN values (all P<0.05, compared with group Q1). In the fully adjusted model II (with all covariates in Table 1 included), the risk of 30-day mortality increased by 5% for every 10 mg/dL increase in the BUN level (HR =1.05; 95% CI: 1.02–1.07, P<0.001) (Table 2). In the subgroup analyses, the risk of death increased progressively in groups Q2, Q3, Q4, and Q5 compared with group Q1, regardless of gender (male or female), age (<65 or ≥65 years), development of heart failure, MV, or use of hypertensors (all P<0.001 in trend tests; Table 3). Kaplan-Meier survival curves and log-rank test showed a significant difference in survival among groups Q1, Q2, Q3, Q4, and Q5 (P<0.001), with the shortest survival time in group Q5 (Figure 2).
Table 2
| Exposure | Nonadjusted | Adjustment I | Adjustment II | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |||
| BUN (mg/dL) | 1.04 (1.01–1.06) | 0.002 | 1.04 (1.02–1.07) | <0.001 | 1.05 (1.02–1.07) | <0.001 | ||
| BUN quintiles | ||||||||
| Q1 [≤12] | Reference | Reference | Reference | |||||
| Q2 [13–18] | 1.32 (1.11–1.56) | 0.001 | 1.17 (0.98–1.39) | 0.074 | 1.12 (0.94–1.33) | 0.194 | ||
| Q3 [19–27] | 1.85 (1.58–2.17) | <0.001 | 1.55 (1.32–1.82) | <0.001 | 1.34 (1.14–1.58) | <0.001 | ||
| Q4 [28–43] | 2.79 (2.39–3.25) | <0.001 | 2.28 (1.95–2.67) | <0.001 | 1.86 (1.58–2.18) | <0.001 | ||
| Q5 [≥44] | 3.90 (3.36–4.52) | <0.001 | 3.23 (2.78–3.76) | <0.001 | 2.49 (2.12–2.93) | <0.001 | ||
| P for trend | <0.001 | <0.001 | <0.001 | |||||
Nonadjusted: no covariates were adjusted; adjustment I model: adjusted for age and gender; adjustment II model: adjusted for all covariates in Table 1. BUN, blood urea nitrogen; HR, hazard ratio; CI, confidence interval.
Table 3
| Confounding factor category | BUN quintiles (mg/dL) | P for trend | P for interaction | ||||
|---|---|---|---|---|---|---|---|
| Q1 [≤12] | Q2 [13–18] | Q3 [19–27] | Q4 [28–43] | Q5 [≥44] | |||
| Gender | 0.052 | ||||||
| Female | 1 (Ref) | 1.25 (0.98–1.58) | 1.51 (1.21–1.89) | 1.71 (1.37–2.15) | 2.67 (2.12–3.35) | <0.001 | |
| Male | 1 (Ref) | 1.07 (0.82–1.40) | 1.26 (0.98–1.63) | 1.99 (1.55–2.55) | 2.43 (1.89–3.12) | <0.001 | |
| Age | 0.121 | ||||||
| <65 | 1 (Ref) | 1.09 (0.84–1.43) | 1.15 (0.88–1.49) | 1.72 (1.33–2.23) | 2.36 (1.83–3.03) | <0.001 | |
| ≥65 | 1 (Ref) | 1.17 (0.92–1.49) | 1.40 (1.12–1.76) | 1.81 (1.45–2.27) | 2.49 (1.98–3.12) | <0.001 | |
| CHF | 0.142 | ||||||
| No | 1 (Ref) | 1.14 (0.93–1.38) | 1.36 (1.12–1.64) | 1.86 (1.54–2.24) | 2.58 (2.14–3.12) | <0.001 | |
| Yes | 1 (Ref) | 1.16 (0.77–1.74) | 1.19 (0.81–1.75) | 1.61 (1.11–2.35) | 1.98 (1.35–2.9) | <0.001 | |
| MV use | 0.597 | ||||||
| No | 1 (Ref) | 1.15 (0.87–1.52) | 1.31 (1.00–1.71) | 1.67 (1.28–2.18) | 2.44 (1.87–3.18) | <0.001 | |
| Yes | 1 (Ref) | 1.16 (0.92–1.45) | 1.35 (1.09–1.68) | 1.87 (1.51–2.32) | 2.36 (1.89–2.94) | <0.001 | |
| Vasopressor use | 0.345 | ||||||
| No | 1 (Ref) | 1.08 (0.88–1.32) | 1.26 (1.04–1.52) | 1.67 (1.38–2.02) | 2.45 (2.01–2.97) | <0.001 | |
| Yes | 1 (Ref) | 1.40 (0.96–2.04) | 1.69 (1.19–2.39) | 2.25 (1.59–3.17) | 2.52 (1.78–3.56) | <0.001 | |
Adjusted for all covariates in Table 1. BUN, blood urea nitrogen; MIMIC-III Medical Information Mart for Intensive Care III; CHF, congestive heart failure; MV, mechanical ventilation.
Nonlinear relationship between BUN and 30-day mortality in septic patients
The multivariate Cox regression model and smoothed curve fitting revealed a nonlinear association between BUN level and 30-day mortality, and the BUN level inflection point was 41.1 mg/dL (Figure 3). Two different slopes were fitted with segmented multivariate Cox regression models. It was found the P value of the likelihood ratio test was below 0.001 (Table 4). Therefore, we used two segmented models to fit the association between BUN level and 30-day mortality. The effect value was 1.298 (HR =1.298; 95% CI: 1.224–1.376, P<0.001) for BUN <41.1 mg/dL; however, the effect value was 1.045 (HR =1.045; 95% CI: 1.016–1.075; P=0.002) for BUN ≥41.1 mg/dL (Table 3).

Table 4
| Threshold of driving pressure | HR | 95% CI | P value |
|---|---|---|---|
| <41.1 (mg/dL) | 1.298 | (1.224, 1.376) | <0.001 |
| ≥41.1 (mg/dL) | 1.045 | (1.016, 1.075) | 0.002 |
| Likelihood ratio test | – | – | <0.001 |
Adjusted for all covariates in Table 1. BUN, blood urea nitrogen; HR, hazard ratio; CI, confidence interval.
Discussion
In this observational, retrospective cohort study, we used the MIMIC-III database to explore the relationship between BUN level and 30-day mortality in patients with sepsis. A nonlinear relationship between BUN level and 30-day mortality was found in these patients. The 30-day mortality rate of septic patients increased as BUN level increased, and this association differed at an inflection point of 41.1 mg/dL. For BUN <41.1 mg/dL, the 30-day mortality rate of septic patients increased by 29.8% for each 10 mg/dL increase in BUN (HR =1.298; 95% CI: 1.224–1.376; P<0.001); however, for BUN ≥41.1 mg/dL, the 30-day mortality rate increased by only 4.5% for each 10 mg/dL increase in BUN (HR =1.045; 95% CI: 1.016–1.075; P=0.002). Thus, early and effective treatment is very important, and BUN can be used as a simple and quick marker for risk stratification in septic patients.
Rapid risk stratification of septic patients is valuable, especially for emergency medical service (EMS) staff. A simple and commonly used indicator can help identify patients at high risk of death early and quickly, allocate medical resources appropriately, and increase the overall success rate of resuscitation for sepsis patients. Currently, there are many tools available for risk stratification of septic patients, among which the APACHE II score is the most widely used (3). However, it involves many parameters and is cumbersome to apply, and incorporates some unstable parameters, such as heart rate and respiratory rate. Other studies have shown that indicators such as elevated plasma acetylcarnitine (14), increased width of red blood cell distribution (15), and increased neutrophil percentage to albumin ratio (16) can be used as predictors of increased risk of death in patients with sepsis. There are also studies that have used machine learning approaches to develop models for predicting the prognosis of critically ill patients, which are expected to improve early mortality prediction and support the clinical decision-making in the ICU for high-risk patients (17,18). This may become a mainstay in the future and has the potential to replace traditional disease scoring; however, there are differences among models, and the large heterogeneity limits the integration of these models. Due to the differences in the level of health care among different countries and regions, it is important to find generic and simple indicators for the rapid risk stratification of septic patients.
Organ dysfunction is a key clinical feature of sepsis (1), and acute kidney injury is a common organ injury in septic patients which increases mortality (7). According to Kidney Disease: Improving Global Outcomes (KDIGO) guidelines (19), AKI was divided into stages 1, 2 and 3, and the severity increased in turn. Correspondingly, the risk of death increased in turn. The adjusted odds ratio for in-hospital mortality was 1.679 (95% CI: 0.890–3.169; P=0.109) for stage 1, 2.945 (95% CI: 1.382–6.276; P=0.005) for stage 2, and 6.884 (95% CI: 3.876–12.228; P<0.001) for stage 3 (20,21). Common indicators used to evaluate renal function include estimation of glomerular filtration rate (eGFR), blood creatinine, BUN and so on (22). Serum creatinine and BUN are excreted through the kidneys, and both increase with the loss of renal function. However, their clinical significance differs due to their different physiological characteristics. Serum creatinine is produced by muscles. In critically ill patients, muscle proteolysis occurs and muscle content decreases, resulting in a lesser increasing extent in serum creatinine (5). Urea nitrogen is a product of protein catabolism. Critically ill patients are in a state of high protein catabolism (23), and the rise in BUN is more pronounced than that of serum creatinine (5), suggesting that BUN has additional predictive value over serum creatinine. A retrospective study from China showed that the incidence of AKI in patients with sepsis was 47.9%, and the 28-day mortality was 32.7%. BUN was an independent risk factor for death (24). Another study analyzed 398 patients with Escherichia coli bacteremia. The 30-day mortality rate was 14.1%, and the increased ratio of BUN to serum albumin (BUN/ALB) was an independent risk factor for 30-day mortality. The ROC curve of the prediction of 30-day mortality by BUN/ALB ratio was drawn, and the area under the ROC curve (AUC) was 0.712 (95% CI: 0.591–0.805; P=0.003), and the optimal cutoff value of the elevated BUN/ALB ratio was 0.3 (67.5% sensitivity and 65.1% specificity) (25). The present study also confirmed that in patients with sepsis, the 30-day mortality rate increased progressively with the increase in BUN. Filippatos et al. found higher baseline BUN to be a powerful predictor of increased postdischarge mortality in patients hospitalized for heart failure, even in the absence of severe renal failure. Even mild to moderate elevations in baseline BUN have been shown to be predictive, and the 60-day mortality was highest in patients with heart failure in the highest quartile of BUN (BUN >40 mg/dL) (26). In our present study, the BUN inflection point was 41.1 mg/dL, as shown by multivariate Cox regression modeling and smoothed curve fitting, and the mortality rate continued to rise at BUN above 41.1 mg/dL, but more slowly. An ideal biomarker for predicting prognosis should satisfy the following criteria: (I) it is produced during a pathophysiological process, (II) the change of its value can reflect the alleviation or aggravation of a specific disease, (III) it can accurately predict the risk of death, and (IV) its detection method is simple and result is stable. Obviously, BUN meets all of these four criteria in septic patients.
In 2017, an estimated 11 million people worldwide died of sepsis (27). In recent years, with the progress of medical technology, the mortality rate of patients with sepsis has decreased year by year. However, from a global point of view, there are still significant regional differences in the number of sepsis deaths, age distribution and so on. In the future, we should actively develop tools for early identification of sepsis, so as to make early diagnosis of sepsis. In addition, it is necessary to strengthen the training of guidelines for the treatment of sepsis and guide doctors to standardize treatment. Our goal is to reduce the mortality of septic patients as much as possible.
Our present study had two advantages. First, it is a real-world study with a large sample size, and all the data analyzed were naturally generated during daily patient care and thus are highly generalizable. Second, the BUN data in this study were obtained from both acute kidney injury patients and chronic kidney disease patients because preadmission renal function data are not available for emergency patients in the real-world settings in most countries, which also allows a good extrapolation of the results.
However, our study also had some limitations. First, it had a number of confounding factors due to its retrospective cohort design. Second, some of the data were missing, resulting in incomplete sample inclusion. For example, there were 2,041 patients without BUN measurements within 24 hours of ICU admission or with extremely abnormal value of the first measurement and 63 patients with the wrong time of death recorded. Third, BUN measurement was not strictly standardized in some cases. For example, errors might have occurred when BUN was measured in different hospitals and on different instruments. Finally, only septic patients were included in this analysis; for nonseptic patients, the association between BUN level and mortality may be different.
Conclusions
There is a nonlinear relationship between BUN and 30-day mortality in septic patients. The 30-day mortality rate increases with the increase in BUN level. However, when a BUN cut-off of 41.1 mg/dL is applied, the septic patients show a large difference in death risk and must be managed distinctly.
Acknowledgments
Funding: This article is fully funded by special funds for science and technology development of the Xinjiang Production and Construction Corps (grant number: 2016AD004).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/apm-21-2937
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apm-21-2937). All authors report that this article was fully funded by special funds for science and technology development of the Xinjiang Production and Construction Corps (grant number: 2016AD004) and the payments were made to Shihezi City People’s Hospital. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 2017;43:304-77. [Crossref] [PubMed]
- Dong LH, Zhang QY, Di NN, et al. Are you using the third definition to diagnose sepsis in clinic?—A survey among Chinese intensivists. Ann Palliat Med 2020;9:2926-32. [Crossref] [PubMed]
- Giamarellos-Bourboulis EJ, Norrby-Teglund A, Mylona V, et al. Risk assessment in sepsis: a new prognostication rule by APACHE II score and serum soluble urokinase plasminogen activator receptor. Crit Care 2012;16:R149. [Crossref] [PubMed]
- Nunnally ME, Ferrer R, Martin GS, et al. The Surviving Sepsis Campaign: research priorities for the administration, epidemiology, scoring and identification of sepsis. Intensive Care Med Exp 2021;9:34. [Crossref] [PubMed]
- Haines RW, Zolfaghari P, Wan Y, et al. Elevated urea-to-creatinine ratio provides a biochemical signature of muscle catabolism and persistent critical illness after major trauma. Intensive Care Med 2019;45:1718-31. [Crossref] [PubMed]
- Klaude M, Mori M, Tjäder I, et al. Protein metabolism and gene expression in skeletal muscle of critically ill patients with sepsis. Clin Sci (Lond) 2012;122:133-42. [Crossref] [PubMed]
- Peerapornratana S, Manrique-Caballero CL, Gómez H, et al. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int 2019;96:1083-99. [Crossref] [PubMed]
- Wernly B, Lichtenauer M, Vellinga NAR, et al. Blood urea nitrogen (BUN) independently predicts mortality in critically ill patients admitted to ICU: A multicenter study. Clin Hemorheol Microcirc 2018;69:123-31. [Crossref] [PubMed]
- Arihan O, Wernly B, Lichtenauer M, et al. Blood Urea Nitrogen (BUN) is independently associated with mortality in critically ill patients admitted to ICU. PLoS One 2018;13:e0191697 [Crossref] [PubMed]
- Johnson AE, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data 2016;3:160035 [Crossref] [PubMed]
- Johnson AE, Stone DJ, Celi LA, et al. The MIMIC Code Repository: enabling reproducibility in critical care research. J Am Med Inform Assoc 2018;25:32-9. [Crossref] [PubMed]
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- Muggeo VMR. Segmented: an R package to fit regression models with broken-line relationships. R News 2008;8:20-5.
- Chung KP, Chen GY, Chuang TY, et al. Increased Plasma Acetylcarnitine in Sepsis Is Associated With Multiple Organ Dysfunction and Mortality: A Multicenter Cohort Study. Crit Care Med 2019;47:210-8. [Crossref] [PubMed]
- Li Y, She Y, Fu L, et al. Association Between Red Cell Distribution Width and Hospital Mortality in Patients with Sepsis. J Int Med Res 2021;49:3000605211004221 [Crossref] [PubMed]
- Gong Y, Li D, Cheng B, et al. Increased neutrophil percentage-to-albumin ratio is associated with all-cause mortality in patients with severe sepsis or septic shock. Epidemiol Infect 2020;148:e87 [Crossref] [PubMed]
- Delgado R, Núñez-González JD, Yébenes JC, et al. Survival in the Intensive Care Unit: A prognosis model based on Bayesian classifiers. Artif Intell Med 2021;115:102054 [Crossref] [PubMed]
- Su L, Xu Z, Chang F, et al. Early Prediction of Mortality, Severity, and Length of Stay in the Intensive Care Unit of Sepsis Patients Based on Sepsis 3.0 by Machine Learning Models. Front Med (Lausanne) 2021;8:664966 [Crossref] [PubMed]
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012;120:c179-84. [PubMed]
- Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 2015;41:1411-23. [Crossref] [PubMed]
- Poston JT, Koyner JL. Sepsis associated acute kidney injury. BMJ 2019;364:k4891. [Crossref] [PubMed]
- Kellum JA, Romagnani P, Ashuntantang G, et al. Acute kidney injury. Nat Rev Dis Primers 2021;7:52. [Crossref] [PubMed]
- Sharma K, Mogensen KM, Robinson MK. Pathophysiology of Critical Illness and Role of Nutrition. Nutr Clin Pract 2019;34:12-22. [Crossref] [PubMed]
- Peng Q, Zhang L, Ai Y, et al. Epidemiology of acute kidney injury in intensive care septic patients based on the KDIGO guidelines. Chin Med J (Engl) 2014;127:1820-6. [PubMed]
- Zou XL, Feng DY, Wu WB, et al. Blood urea nitrogen to serum albumin ratio independently predicts 30-day mortality and severity in patients with Escherichia coli bacteraemia. Med Clin (Barc) 2021;157:219-25. [Crossref] [PubMed]
- Filippatos G, Rossi J, Lloyd-Jones DM, et al. Prognostic value of blood urea nitrogen in patients hospitalized with worsening heart failure: insights from the Acute and Chronic Therapeutic Impact of a Vasopressin Antagonist in Chronic Heart Failure (ACTIV in CHF) study. J Card Fail 2007;13:360-4. [Crossref] [PubMed]
- Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet 2020;395:200-11. [Crossref] [PubMed]
(English Language Editor: J. Gray)