
Exploring the effect of microecological agents on postoperative immune function in patients undergoing liver cancer surgery: a systematic review and meta-analysis
Introduction
Primary carcinoma of the liver (PLC), also referred to as hepatocellular carcinoma, is an epithelial malignancy that originates in the liver, of which more than 90% are hepatocellular carcinomas and the rest are cholangiocellular and mixed hepatocellular carcinomas (1). The etiology of primary hepatocellular carcinoma is not fully established, but may be related to viral hepatitis, aflatoxins, metabolic factors, genetic factors, and long-term alcohol and tobacco consumption (2). Patients with early stage liver cancer usually do not have obvious symptoms, but over time, patients may develop symptoms such as pain in the liver area, weakness, lack of appetite, and wasting (3). At present, surgery is considered to be the preferred treatment for liver cancer. The common surgical procedures for liver transplantation include hepatectomy and liver transplantation. Hepatectomy is the removal of the liver tumor and part of the surrounding liver tissue, which is suitable for those with good liver function and involves complete tumor removal during surgery (4).
Recent studies have shown that after liver resection in patients with liver cancer, a series of postoperative problems such as damage to the intestinal barrier, bacterial translocation, liver damage, and endotoxin translocation will occur in patients with liver cancer. Patients will experience varying degrees of oxidative stress after surgery, which will lead to varying degrees of damage to the intestinal mucosal barrier. If the body’s stress response is excessive or dysregulated after liver resection in patients with liver cancer, endotoxins released by conditioned pathogens will cross the intestinal mucosal barrier in some way. A large amount of invasion of tissues other than the intestinal tract, which is normally sterile, will result in postoperative infection.
In patients with primary liver cancer with enema, routine enema before surgery and decreased bile secretion after surgery can cause changes in intestinal acidity and alkalinity, which can lead to imbalance of the flora in the patient’s body (5). At the same time, the antibiotics used to prevent infection after the operation will kill the sensitive bacteria in the intestinal tract, making the resistant bacteria colonize in large numbers, destroying the normal distribution of the intestinal flora in the patient’s body (6). Fasting after liver resection in patients will lead to a reduction in related amino acids and energy intake, which in turn induces a decrease in the permeability of the intestinal mucosa and a weakened barrier function (7). Intestinal flora imbalance and weakened intestinal barrier function have increased the incidence of complications after liver resection. Therefore, the postoperative nutritional supply of PLC patients will directly affect the postoperative recovery of patients.
In recent years, related studies on the nutritional supply of patients with PLC have confirmed that as a microecological regulator of the normal microbiota of the gastrointestinal tract, Microecological agents have a good effect in regulating the microecological balance of the intestinal flora of patients. At present, it has been used clinically in the prevention of colon cancer, acute pancreatitis, severe trauma patients and gastrointestinal fistula after rectal surgery. It has been proven that microbial preparations can significantly alleviate postoperative intestinal flora imbalance, intestinal barrier function damage, endotoxemia and hyperbilirubinemia caused by liver resection, and reduce liver damage to patients. There have been many reports on the use of Microecological agents for the prevention of endotoxemia and liver function damage after liver resection in PLC patients. It has been reported that Microecological agents can well help the resident flora in the gastrointestinal tract to compete for the adsorption sites of the intestinal epithelium, and can inhibit the colonization or reproduction of foreign microorganisms in the intestine (8,9), thereby reducing the risk of postoperative infection and complications of patients.
Therefore, relevant articles related to the use of Microecological agents in patients with PLC were selected for meta-analysis to evaluate the impacts of Microecological agents on the immune function of patients with liver cancer, aiming to provide a reference for the clinical application of microbial agents after hepatectomy. We present the following article in accordance with the PRISMA reporting checklist (available at https://dx.doi.org/10.21037/apm-21-2669).
Methods
Literature search
The China National Knowledge Internet (CNKI) (1979–2021.4), China Biomedical Literature Database (1994–2021.4), Cochrane Library (2005–2021.4), Medline (1948–2021.4), Embase (1966.1–2021.4), and other databases were electronically searched for relevant published randomized controlled trials (RCTs) on the use of microecological agents after liver surgery. Also, relevant literature in journals was hand-searched. Relevant literature was selected using a compound Boolean logical search. Chinese and English databases were searched using the following search terms: “hepatocellular carcinoma”, “hepatectomy”, “microecological agents”, and “microecological regulators”. The meta-analysis was performed using Rev Man 5.3 and Stata 13 software provided by the Cochrane system.
The above search terms were freely combined, and the first screening of the initially-retrieved literature was performed by reading the titles and abstracts to exclude non-compliant articles and to identify relevant studies. A second screening was performed based on the inclusion and exclusion criteria, and the included literature was traced using a search engine. Finally, a third screening was performed to evaluate the quality of the articles by reading their full texts. The deadline for retrieval of all documents was June 10, 2021.
Literature inclusion/exclusion criteria
The inclusion criteria were as follows: (I) published RCTs of blank controls versus microecological agents for the prevention of post-hepatectomy complications; (II) research involving study subjects with a confirmed diagnosis of hepatocellular carcinoma and a child grade of A or B; and (III) studies involving microecological agents of type as probiotic dairy products or drugs, used for >7 days.
The exclusion criteria were as follows: (I) literature such as conference presentations, review articles, research reports, and lectures; (II) non-clinical RCTs; (III) JADAD score ≤2; and (IV) literature with inaccessible full text, incomplete data, or duplicate publications.
Observed indicators
The main indicators in this study were as follows: glutathione transaminase level, glutathione alanine transaminase level, interleukin-6 (IL-6) level, total bilirubin (TBIL) level, direct bilirubin (DBIL) level, and peripheral blood endotoxin level.
Data extraction
Data were extracted independently by two experts using Microsoft Excel, and consistent conclusions were obtained through discussion in cases of disagreement between the two experts. The following data was extracted from the included studies: authors, title, publication date, sample size, treatment protocol, evaluation index, duration of treatment, and efficacy. The quality of the literature was evaluated using JADAD scores based on the following criteria: (I) the study was randomized; (II) the randomization method was correct; (III) the study was double-blinded; and (IV) the description of double-blind hair. Each of the aforementioned aspects was scored 1 point; a total score ≤2 was considered a low-quality study, while a total score ≥3 was considered a high-quality study.
Risk of bias and quality assessment
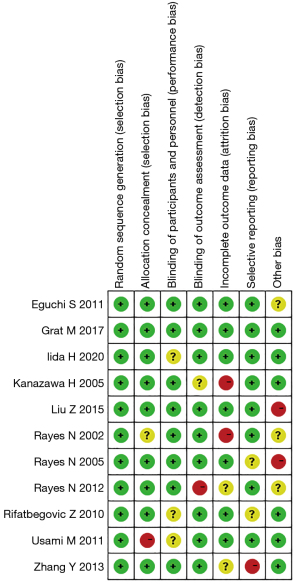
The literature was independently screened by two experts according to the inclusion and exclusion criteria. The Cochrane Handbook Risk of Bias Assessment Tool for Randomized Controlled Trials was used to evaluate the risk of bias for the inclusion of RCTs. Specifically, the RCTs were evaluated based on whether the random assignment method was correct, whether the allocation protocol was concealed, whether the method was correct, selective reporting of study results, completeness of study data, as well as whether the study subjects, treatment protocols, and study results were blinded. The above entries were judged as “high risk of bias”, “low risk of bias”, and “unclear”, respectively.
Statistical analysis
Statistical analysis was performed using RevMan 5.3 software. The risk of bias assessment chart in RevMan 5.3 software was adopted to assess the risk bias of the included articles. The results of each included study were tested for heterogeneity by using the χ2 test, in which continuous variable results were expressed as mean difference (MD) or standardized mean difference (SMD), and discontinuous variables were expressed as odds ratio (OR). All effects were expressed with a 95% confidence interval (CI). When the heterogeneity results P>0.01 and I2<50%, it meant that the homogeneity of the results of each study was high, and the fixed-effects model was used for meta-analysis. When P<0.01 and I2>50%, it showed that the heterogeneity of the results of each study was high, and the random effects model was used for meta-analysis. If the heterogeneity was too large, it was necessary to further carry out the sensitivity index of the index.
Results
Search results and basic information of the included literature
A total of 1,357 articles were screened in this study, of which 70 articles were repeatedly published, 81 articles were marked as unqualified by automated tools, and 86 articles were eliminated due to other reasons. After the abstracts and titles of the articles were read, 158 articles were eliminated, leaving 962 articles. After the full texts were read, 713 review articles and 217 research reports were excluded, leaving 32 articles. After 12 articles with non-randomized controlled experiments and 9 articles with insufficient observation indicators were excluded, 11 articles were selected (Figure 1).
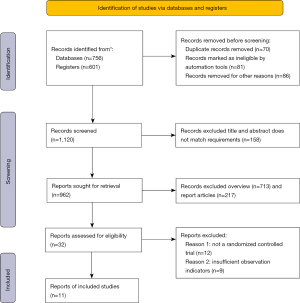
There were 11 articles (10-20) that met the inclusion criteria, and there were 788 patient cases. Among the 11 articles, they were all small-sample studies, with the sample size ranging from 9 to 68, and the age of the research subjects was all over 45 years old. The 11 articles described the sample size, experimental grouping, treatment plan, course of treatment, and JADAD score in detail. Table 1 showed the basic characteristics of articles included.
Table 1
| Author | Year of publication | Number of cases | Treatment protocol | Treatment course | JADAD rating | |||
|---|---|---|---|---|---|---|---|---|
| Microecological preparation group | Control group | Microecological preparation group | Control group | |||||
| Eguchi S (10) | 2011 | 25 | 25 | Synbiotics | No intervention, enteric nutrition | 16 | 3 | |
| Grąt M (11) | 2017 | 21 | 23 | Probiotics | Placebo | 30/90 | 4 | |
| Iida H (12) | 2020 | 60 | 60 | Clostridium butyricum and fibers | No intervention | <14, 14–70, >70 | 3 | |
| Kanazawa H (13) | 2005 | 21 | 23 | Bifidobacterium breve strain Yakult, Lactobacillus casei strain Shirota; prebiotic: galactooligo saccharides | No intervention, enteric nutrition | 13 | 2 | |
| Liu Z (14) | 2015 | 66 | 68 | Lactobacillus plantarum, Lactobacillus acidophilus, Bifidobacterium longum | Placebo | 16 | 3 | |
| Rayes N (15) | 2002 | 31 | 32 | Synbiotics | Placebo/inulin | 13 | 4 | |
| Rayes N (16) | 2005 | 33 | 33 | Synbiotics | Placebo/fibers | 13 | 3 | |
| Rayes N (17) | 2012 | 9 | 10 | Pediacoccus pentosaceus, Leuconostoc mesenteroides Lactobacillus paracasei subspecies paracasei, Lactobacillus plantarum; prebiotic: bioactive fibers: betaglucan, inulin, pectin, and resistant starch | Placebo/fibers | 11 | 4 | |
| Rifatbegovic Z (18) | 2010 | 60 | 60 | Lactobacillus plantarum 2,362, L. paracasei subsp paracasei 19, Pediacoccus pentoseceus 5–33:3 and 32–77:1, L. raffinolactis and fibers | No intervention | 10 | 2 | |
| Usami M (19) | 2011 | 32 | 29 | Lactobacillus casei strain Shirota, Bifidobacterium breve strain Yakult; prebiotic: galactooligosaccharides | No intervention | 25 | 3 | |
| Zhang Y (20) | 2013 | 34 | 33 | Synbiotics | Enteric nutrition and fibers | 7 | 4 | |
Risk of bias evaluation results of the included studies
Figures 2,3 showed the results of multiple risk bias evaluations of included articles drawn by Rev Man 5.3 software. In this study, among the 12 randomized controlled experiments, 11 described the correct random allocation method, accounting for 100%, and 8 described the hidden allocation plan in detail, accounting for 72.72%. There were 9 articles using blind method, accounting for 81.81%, and no blind method was used in the remaining articles.
Meta-analysis of the effects of microecological agents on patients’ alanine aminotransferase (ALT)
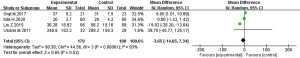
Figure 4 was a forest diagram of the effects of Microecological agents on ALT in patients with liver cancer. Among the 11 articles included in the study, 4 articles described in detail the changes in the ALT levels of the Microecological agent group and the control group. The relevant data of the 4 articles were extracted to analyze the heterogeneity of the ALT levels of patients with liver cancer surgery. The results showed that chi-square test (Chi2) =44.56, degrees of freedom (df) =3, I2=93% >50%, and P<0.00001, indicating obvious heterogeneity. Statistical analysis using a random effects model showed that there was no significant difference between Microecological agents and placebo treatment in the control group on the ALT levels of patients with liver cancer after hepatectomy [MD =−3.65, 95% CI: (−14.65, 7.34), P=0.52].
Meta-analysis of the effect of microecological preparations on aspartate transaminase (AST) in patients
Figure 5 was a forest diagram of the effects of Microecological agents on AST in patients with liver cancer. Among the 11 articles included in the study, 4 articles described in detail the changes in the AST levels of the Microecological agent group and the control group. The relevant data of the 4 articles were extracted to analyze the heterogeneity of the AST levels of patients with liver cancer surgery. The results showed that Chi2=9.03, df=3, I2=67% >50%, and P=0.03, indicating obvious heterogeneity among the included articles. Statistical analysis using a random effects model showed that there was no significant difference between Microecological agents and placebo treatment in the control group on the AST levels of patients with liver cancer after hepatectomy [MD =−0.64, 95% CI: (−6.87, 5.58), P=0.84].

Meta-analysis of the impacts of Microecological agents on the prothrombin level of patients
Figure 6 was a forest diagram of the effects of Microecological agents on prothrombin level in patients with liver cancer. Among the 11 articles included in the study, 2 articles described in detail the changes in the prothrombin level of the Microecological agent group and the control group. The relevant data of the 2 articles were extracted to analyze the heterogeneity of the prothrombin level of patients with liver cancer surgery. The results showed that Chi2=0, df=7, I2=0% <50%, and P=1, indicating good homogeneity among the included articles. Statistical analysis using a random effects model showed that there was no significant difference between Microecological agents and placebo treatment in the control group on the prothrombin level of patients with liver cancer after hepatectomy [MD =1, 95% CI: (−2.57, 4.57), P=0.58].
Meta-analysis of the impacts of Microecological agents on total bilirubin (TBIL) levels
Figure 7 was a forest diagram of the effects of Microecological agents on TBIL level in patients with liver cancer. Among the 11 articles included in the study, 3 articles described in detail the changes in the TBIL level of the Microecological agent group and the control group. The relevant data of the 2 articles were extracted to analyze the heterogeneity of the TBIL level of patients with liver cancer surgery. The results showed that Chi2=1.23, df=2, I2=0% <50%, and P=0.54, indicating good homogeneity among the included articles. Statistical analysis using a random effects model showed that Microecological agents could greatly lower the TBIL contents of patients with liver cancer after hepatectomy [MD =−0.10, 95% CI: (−0.14, −0.06), P<0.00001].
Meta-analysis of the impacts of Microecological agents on patients’ CRP
Figure 8 was a forest diagram of the effects of Microecological agents on CRP level in patients with liver cancer. Among the 11 articles included in the study, 2 articles described in detail the changes in the CRP level of the Microecological agent group and the control group. The relevant data of the 2 articles were extracted to analyze the heterogeneity of the CRP level of patients with liver cancer surgery. The results showed that Chi2=0.02, df=1, I2=0% <50%, and P=0.88, indicating obvious heterogeneity among the included articles in term of CRP level. Statistical analysis using a random effects model showed that Microecological agents could greatly lower the CRP contents of patients with liver cancer after hepatectomy [MD =−0.28, 95% CI: (−3.01, 2.45), P=0.84].

Meta-analysis of impacts of microecological agents on the incidence of postoperative infections in patients
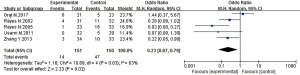
Figure 9 was a forest diagram of the effects of Microecological agents on incidence of postoperative infections in patients with liver cancer. Among the 11 articles included in the study, 5 articles described in detail the changes in the incidence of postoperative infections of the Microecological agent group and the control group. The relevant data of the 5 articles were extracted to analyze the heterogeneity of the incidence of postoperative infections of patients with liver cancer surgery. The results showed that Chi2=10.69, df=4, I2=63% >50%, and P=0.03, indicating obvious heterogeneity among the included articles in term of incidence of postoperative infections. Statistical analysis using a random effects model showed that Microecological agents could greatly lower the incidence of postoperative infections of patients with liver cancer after hepatectomy [MD =0.23, 95% CI: (0.07, 0.79), P=0.02 <0.05].
Meta-analysis of the impacts of microecological agents on the incidence of postoperative complications in patients
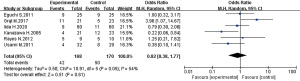
Figure 10 was a forest diagram of the effects of Microecological agents on incidence of postoperative complications in patients with liver cancer. Among the 11 articles included in the study, 6 articles described in detail the changes in the incidence of postoperative complications of the Microecological agent group and the control group. The relevant data of the 6 articles were extracted to analyze the heterogeneity of the incidence of postoperative complications of patients with liver cancer surgery. The results showed that Chi2=10.91, df=5, I2=54% >50%, and P=0.05, indicating obvious heterogeneity among the included articles in term of incidence of postoperative complications. Statistical analysis using a random effects model showed that the incidence of postoperative complications patients with liver cancer in the experimental group and control group was not statically obvious after hepatectomy [OR =0.82, 95% CI: (0.38, 1.77), P=0.61].
Publication bias results
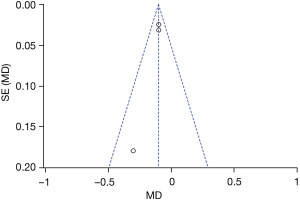
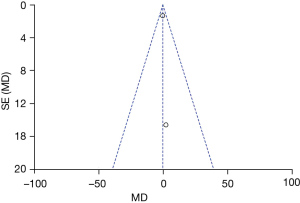
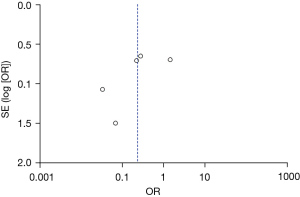
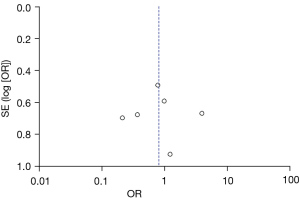
Figures 11-17 showed the funnel charts of publication bias of the included articles. Among them, Figures 11-17 were funnel charts based on the data of AST, ALT, prothrombin, bilirubin, CRP, postoperative infection, and postoperative complications, respectively. Some of the circles included in the study were basically concentrated on the midline and are basically symmetrical to the midline, indicating that there was no publication bias in the included articles. Therefore, the conclusions drawn were relatively reliable.
Discussion
This systematic review study included 11 articles, which were designed to evaluate the effects of Microecological agents on postoperative immune function of patients with hepatocellular carcinoma. All 11 articles described the correct random allocation method, 8 detailed the concealment of the allocation plan, and 9 articles used the blind method. The reason may be the unclear random allocation method of the study or the subjective bias of doctors (21).
The analysis of the heterogeneity of postoperative ALT levels of patients undergoing liver cancer surgery in this study showed that there was a certain heterogeneity among the postoperative ALT levels of liver cancer patients included in the study. The statistical analysis of random effects model showed that there was no significant difference between the postoperative ALT levels of liver cancer patients treated with microbial agents and placebo [MD =−3.65, 95% CI: (−14.65, 7.34), P=0.52]. There was also no significant difference in AST levels between the two groups of patients included in these 4 articles [MD =−0.64, 95% CI: (−6.87, 5.58), P=0.84]. Such results were different from the conclusions of many similar studies that microbial preparations can significantly reduce postoperative ALT and AST levels in patients with liver cancer compared with placebo treatment (22). This can be due to the fact that the number of related studies involving Microecological agents used for the postoperative treatment of liver cancer patients included in this study was too small, and there was a certain difference between the postoperative ALT level and AST level data of the included liver cancer patients.
In addition, the analysis of the heterogeneity of the levels of prothrombin, bilirubin, and CRP in patients with liver cancer showed that the differences in the levels of prothrombin between the Microecological agent group and the control group were not statistically significant [MD =1, 95% CI: (−2.57, 4.57), P=0.58]. There was no significant difference in CRP content between the experimental group and the control group in patients with liver cancer after liver cancer surgery, and it was not statistically significant [MD =−0.28, 95% CI: (−3.01, 2.45), P=0.84]. The comparison of TBIL levels in patients with liver cancer after surgery showed that microbial agents significantly reduced the TBIL levels in patients after hepatectomy [MD =−0.10, 95% CI: (−0.14, −0.06), P<0.00001]. This shows that the use of microbial agents can significantly reduce liver damage after hepatectomy for primary hepatocellular carcinoma, repair the intestinal barrier damage, relieve the symptoms of intestinal flora imbalance, and reduce the occurrence of postoperative hyperbilirubinemia (23). Current research shows that Microecological agents can reduce the probability of postoperative complications in patients undergoing liver resection because they can antagonize non-native bacteria, stimulate intestinal mucosal proliferation, promote the absorption of nutrients in the intestine, and increase the vitality of the patient’s immune system (24). Among them, the impact of Microecological agents on the immune system is mainly reflected in the promotion of normal intestinal flora to stimulate the host’s immune system through the bacteria itself or through cell wall components, activate immune cells, and exert their effects on the surface through the production of antibodies, interferons, interleukins, etc. (25).
In addition, comparison on the results of meta-analysis involving postoperative infection and complication rates of liver cancer patients included in the articles revealed that compared with placebo treatment, treatment with Microecological agents can significantly reduce the risk of postoperative infection in patients with liver cancer [MD =0.23, 95% CI: (0.07, 0.79), P=0.02<0.05]. The meta-analysis results of the incidence of postoperative complications of liver cancer patients included in articles showed that the difference between the incidence of postoperative complications of liver cancer patients in the experimental group and the control group was not statistically significant [OR =0.82, 95% CI: (0.38, 1.77), P=0.61]. Summary on similar studies reveals that the possible mechanisms for microbial agents to reduce the probability of infection and complications after liver resection include the following aspects. Firstly, the antagonistic effect of resident bacteria in the body on non-native bacteria. Secondly, microbial preparations can stimulate glycolysis. Thirdly, microbial preparations can stimulate the proliferation of intestinal mucosal cells. Fourthly, microbial preparations activate immune cells, produce corresponding immune active factors (antibodies, interferons, interleukins, etc.), and improve the vitality of the immune system (26). Fifthly, it could increase intestinal absorption of vitamin nutrients (B vitamins, vitamin K, vitamin C, niacin, biotin, folic acid, etc.). Finally, some components in microbial preparations have anti-tumor effects, for example, bifidobacteria can reduce the pH of the intestinal lumen, inhibit the formation of carcinogens, or activate macrophages to play an anti-tumor effect (27,28). The above-mentioned various reasons may lead to the effect of Microecological agents to reduce the risk of postoperative infection in patients with liver cancer and enhance the immunity of patients.
For a meta-analysis of the effects of Microecological agents on the immune function of patients after liver cancer surgery, the funnel chart showed that the published articles included in this study were not biased. Therefore, the conclusions obtained were credible, and risk bias was not the main factor affecting the conclusions.
Conclusions
This study included 11 articles on the use of Microecological agents to provide postoperative nutrition for patients undergoing liver cancer surgery. It was confirmed by meta-analysis that Microecological agents can significantly reduce the bilirubin level of patients after hepatectomy. It also significantly reduces the risk of postoperative infection in patients with liver cancer, and significantly improves the immune function of patients with liver cancer. The limitations of this study were as follows. Firstly, the articles lack some important biochemical indicators. Secondly, there are information biases in the data collection process. Thirdly, changing the inclusion criteria and excluding low-quality studies can’t significantly reduce heterogeneity. There was a certain degree of heterogeneity in the included articles, which may have a certain impact on the results. At the same time, the Ben study did not fully evaluate the safety of the Microecological agents in the articles. Therefore, further research was needed in the future. In short, this study provided some scientific references for postoperative recovery treatment of liver cancer patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://dx.doi.org/10.21037/apm-21-2669
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apm-21-2669). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Maulat C, Regimbeau JM, Buc E, et al. Prevention of biliary fistula after partial hepatectomy by transcystic biliary drainage: randomized clinical trial. Br J Surg 2020;107:824-31. [Crossref] [PubMed]
- Wang H, Chen G, Gao H, et al. Successful treatment of acute tumor lysis syndrome associated with transcatheter chemoembolization with large hepatocellular carcinomas: two case reports. Transl Cancer Res 2020;9:6516-21. [Crossref]
- Dello SA, Reisinger KW, van Dam RM, et al. Total intermittent Pringle maneuver during liver resection can induce intestinal epithelial cell damage and endotoxemia. PLoS One 2012;7:e30539 [Crossref] [PubMed]
- Orcutt ST, Anaya DA. Liver Resection and Surgical Strategies for Management of Primary Liver Cancer. Cancer Control 2018;25:1073274817744621 [Crossref] [PubMed]
- Boudjema K, Locher C, Sabbagh C, et al. Simultaneous Versus Delayed Resection for Initially Resectable Synchronous Colorectal Cancer Liver Metastases: A Prospective, Open-label, Randomized, Controlled Trial. Ann Surg 2021;273:49-56. [Crossref] [PubMed]
- Scherman P, Syk I, Holmberg E, et al. Influence of primary tumour and patient factors on survival in patients undergoing curative resection and treatment for liver metastases from colorectal cancer. BJS Open 2020;4:118-32. [Crossref] [PubMed]
- Fenton HM, Taylor JC, Lodge JPA, et al. Variation in the Use of Resection for Colorectal Cancer Liver Metastases. Ann Surg 2019;270:892-8. [Crossref] [PubMed]
- Rayes N, Seehofer D, Müller AR, et al. Influence of probiotics and fibre on the incidence of bacterial infections following major abdominal surgery - results of a prospective trial. Z Gastroenterol 2002;40:869-76. [Crossref] [PubMed]
- Seguin P, Locher C, Boudjema K, et al. Effect of a Perioperative Nutritional Supplementation with Oral Impact® in Patients undergoing Hepatic Surgery for Liver Cancer: A Prospective, Placebo-Controlled, Randomized, Double-Blind Study. Nutr Cancer 2016;68:464-72. [Crossref] [PubMed]
- Eguchi S, Takatsuki M, Hidaka M, et al. Perioperative synbiotic treatment to prevent infectious complications in patients after elective living donor liver transplantation: a prospective randomized study. Am J Surg 2011;201:498-502. [Crossref] [PubMed]
- Grąt M, Wronka KM, Lewandowski Z, et al. Effects of continuous use of probiotics before liver transplantation: A randomized, double-blind, placebo-controlled trial. Clin Nutr 2017;36:1530-9. [Crossref] [PubMed]
- Iida H, Sasaki M, Maehira H, et al. The effect of preoperative synbiotic treatment to prevent surgical-site infection in hepatic resection. J Clin Biochem Nutr 2020;66:67-73. [Crossref] [PubMed]
- Kanazawa H, Nagino M, Kamiya S, et al. Synbiotics reduce postoperative infectious complications: a randomized controlled trial in biliary cancer patients undergoing hepatectomy. Langenbecks Arch Surg 2005;390:104-13. [Crossref] [PubMed]
- Liu Z, Li C, Huang M, et al. Positive regulatory effects of perioperative probiotic treatment on postoperative liver complications after colorectal liver metastases surgery: a double-center and double-blind randomized clinical trial. BMC Gastroenterol 2015;15:34. [Crossref] [PubMed]
- Rayes N, Seehofer D, Hansen S, et al. Early enteral supply of lactobacillus and fiber versus selective bowel decontamination: a controlled trial in liver transplant recipients. Transplantation 2002;74:123-7. [Crossref] [PubMed]
- Rayes N, Seehofer D, Theruvath T, et al. Supply of pre- and probiotics reduces bacterial infection rates after liver transplantation--a randomized, double-blind trial. Am J Transplant 2005;5:125-30. [Crossref] [PubMed]
- Rayes N, Pilarski T, Stockmann M, et al. Effect of pre- and probiotics on liver regeneration after resection: a randomised, double-blind pilot study. Benef Microbes 2012;3:237-44. [Crossref] [PubMed]
- Rifatbegovic Z, Mesic D, Ljuca F, et al. Effect of probiotics on liver function after surgery resection for malignancy in the liver cirrhotic. Med Arh 2010;64:208-11. [PubMed]
- Usami M, Miyoshi M, Kanbara Y, et al. Effects of perioperative synbiotic treatment on infectious complications, intestinal integrity, and fecal flora and organic acids in hepatic surgery with or without cirrhosis. JPEN J Parenter Enteral Nutr 2011;35:317-28. [Crossref] [PubMed]
- Zhang Y, Chen J, Wu J, et al. Probiotic use in preventing postoperative infection in liver transplant patients. Hepatobiliary Surg Nutr 2013;2:142-7. [PubMed]
- Sugawara G, Nagino M, Nishio H, et al. Perioperative synbiotic treatment to prevent postoperative infectious complications in biliary cancer surgery: a randomized controlled trial. Ann Surg 2006;244:706-14. [Crossref] [PubMed]
- Okabayashi T, Iyoki M, Sugimoto T, et al. Oral supplementation with carbohydrate- and branched-chain amino acid-enriched nutrients improves postoperative quality of life in patients undergoing hepatic resection. Amino Acids 2011;40:1213-20. [Crossref] [PubMed]
- Cornide-Petronio ME, Álvarez-Mercado AI, Jiménez-Castro MB, et al. Current Knowledge about the Effect of Nutritional Status, Supplemented Nutrition Diet, and Gut Microbiota on Hepatic Ischemia-Reperfusion and Regeneration in Liver Surgery. Nutrients 2020;12:284. [Crossref] [PubMed]
- Micó-Carnero M, Rojano-Alfonso C, Álvarez-Mercado AI, et al. Effects of Gut Metabolites and Microbiota in Healthy and Marginal Livers Submitted to Surgery. Int J Mol Sci 2020;22:44. [Crossref] [PubMed]
- Sun Y, Yang Z, Tan H. Perioperative nutritional support and fluid therapy in patients with liver diseases. Hepatobiliary Surg Nutr 2014;3:140-8. [PubMed]
- Woo TDH, Oka K, Takahashi M, et al. Inhibition of the cytotoxic effect of Clostridium difficile in vitro by Clostridium butyricum MIYAIRI 588 strain. J Med Microbiol 2011;60:1617-25. [Crossref] [PubMed]
- Li L, Liu C, Yang J, et al. Early postoperative controlling nutritional status (CONUT) score is associated with complication III-V after hepatectomy in hepatocellular carcinoma: A retrospective cohort study of 1,334 patients. Sci Rep 2018;8:13406. [Crossref] [PubMed]
- Gong Y, Liu Z, Liao Y, et al. Effectiveness of ω-3 Polyunsaturated Fatty Acids Based Lipid Emulsions for Treatment of Patients after Hepatectomy: A Prospective Clinical Trial. Nutrients 2016;8:357. [Crossref] [PubMed]
(English Language Editor: A. Kassem)





