
Effect of low-dose esketamine on pain control and postpartum depression after cesarean section: a retrospective cohort study
Introduction
Elective or emergency cesarean section (CS) is one of the most common operations, accounting for approximately 25% to 45% of all births in China (1-4). Although a large number of pregnant women undergo CS, which provides significant management experience, the postoperative nursing of these pregnant women is still a challenge. The most important problem is postoperative pain control after CS, including pain caused by surgical incision and uterine contraction (5). Persistent postoperative pain can lead to maternal inability to discharge respiratory secretions, intestinal obstruction, and venous thrombosis caused by long-term bed rest (6). In addition, postoperative pain will also affect the secretion of milk, delay the milk feeding of newborns, and reduce the intimate communication between mothers and infants (7). More seriously, long-term chronic pain may lead to postpartum depression (PPD). A previous study indicated that postoperative pain was correlated with the early onset of PPD after CS (8). PPD is present in 10–20% of pregnant women, mostly within 1 year after delivery (9-11). PPD significantly affects the mother’s quality of recovery, and can delay the baby’s growth and development due to the deterioration of the mother-infant relationship. In the most serious cases, PPD can lead to maternal suicide. It has been reported that PPD is associated with about 20% of maternal postoperative deaths (12). Therefore, the proper management of postoperative pain should be further explored to improve the quality of recovery of pregnant women undergoing CS.
The standard treatment of postoperative pain after CS is the use of opioids, which can exert a rapid analgesic effect for a long time, but may also lead to some adverse reactions (13). Nowadays, many drugs are used in combination to reduce the use of opioids, maintain effective analgesia, and reduce the incidence of adverse reactions. Esketamine, an enantiomer of ketamine, acts as a non-selective N-methyl-D-aspartic acid (NMDA) receptor inhibitor. Esketamine was initially used as a psychotropic drug for treatment-resistant depression, which was reported to improve the functional outcomes of patients (14). In recent years, some studies have also begun to use esketamine to control postoperative pain and reduce the use of opioids. Nielsen et al. reported that intraoperative esketamine could reduce postoperative pain and opioid use in patients undergoing spinal surgery (15). Furthermore, Liu et al. reported that esketamine was effective for reducing postoperative depression in breast cancer patients without increasing the incidence of adverse events (16). However, no study to date has been performed to determine the effects of esketamine on pain control as well as PPD in pregnant women undergoing CS. This retrospective cohort study was conducted in a single center to explore the effects of esketamine by comparing postoperative pain and PPD after CS between pregnant women receiving esketamine and those not receiving esketamine. Meanwhile, subgroup analysis was also performed to compare the effects of different doses of esketamine. We present the following article in accordance with the STROBE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-21-3343/rc).
Methods
Study design
This study was performed in Wuxi No. 9 People’s Hospital Affiliated to Soochow University. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Wuxi No. 9 People’s Hospital Affiliated to Soochow University (No. 0021022). Individual consent for this retrospective analysis was waived.
Patient selection
Pregnant women who had undergone CS between March 2018 and February 2020 at our hospital were retrospectively reviewed by 2 independent investigators. The following pregnant women were included in this study: (I) those older than 18 years; (II) those with American Society of Anesthesiologists class II; (III) those who received spinal anesthesia; (IV) those who underwent elective or emergency CS; and (V) those who received patient controlled intravenous analgesia (PCIA) using the combination of sufentanil citrate and palonosetron hydrochloride with/without esketamine after CS. The following pregnant women were excluded from this study: (I) those with a psychotic disorder; (II) those allergic to esketamine; (III) those who did not receive PCIA including sufentanil citrate and palonosetron hydrochloride; (IV) those who received combined spinal-epidural anesthesia; (V) those with life-threatening chronic comorbid diseases; and (VI) those who participated in other clinical studies. After including pregnant women, the following demographic and clinical data were collected: age, pre-pregnancy body mass index (BMI), gestational weeks (full term, preterm, and post term), pregnancy complications (gestational diabetes, gestational hypertension, acute fatty liver of pregnancy, placenta previa, and placental abruption), gravidity, parity, prior history of CS, marital status, educational level, employment, pressure during pregnancy, type of CS (elective or emergency), operation time, anesthesia time, volume of blood loss, blood transfusion, fetus, and dose of esketamine. The pressure during pregnancy was classified as high, moderate, and low according to a previous study (17). These data were also collected and discussed by 2 independent investigators.
Procedures
Spinal anesthesia was performed using 12 mg bupivacaine hydrochloride in L2-L3 or L3-L4 subarachnoid spaces before the CS procedure. The anesthesia block level was controlled below T6, and vital signs of parturients were routinely monitored. PCIA was attached to all parturients after CS. The PCIA protocol in the control group consisted of sufentanil citrate 50 µg and palonosetron hydrochloride 0.25 mg in 200 mL saline, while the PCIA protocol in the experimental group consisted of sufentanil citrate 50 µg, palonosetron hydrochloride 0.25 mg, and esketamine 0.2–0.5 mg/kg in 200 mL saline. The infusion rate was 4 mL/h, the bolus dose was 4 mL, and the lockout time was 30 min. All parturients were taught to use PCIA properly in wards, and it was recommended that PCIA be used at least 24 hours after CS. A subgroup analysis was then performed to divide parturients receiving esketamine into a high-dose group (>0.3 mg/kg) and low-dose group (≤0.3 mg/kg) and determine the effects of different doses of esketamine on pain control and PPD.
Outcomes
All parturients were followed up for 3 months and those who were lost to follow-up were excluded from this study. The primary outcomes in this study included postoperative pain control and the incidence of PPD. Pain control was assessed using the numeric rating scale (NRS), which ranged from 0 (painless) to 10 points (most severely painful). PPD was assessed by the Edinburgh postnatal depression scale (EPDS) at 4–6 weeks postpartum and at 3 months postpartum. The EPDS contained 10 questions, with a total score of 30. A total score higher than 9 indicated the existence of PPD in the parturient and timely comprehensive interventions were needed. The secondary outcomes included analgesia-related adverse events (nausea and vomiting, dizziness, and pruritus), sleep quality, cumulative morphine consumption, postpartum anxiety, and quality of recovery. Postpartum anxiety was assessed by the generalized anxiety disorder-7 (GAD-7) scale, with total scores of 21 and higher indicating the existence of postpartum anxiety. The quality of recovery was assessed by a 15-item quality of recovery questionnaire (QoR-15), and higher scores indicated better quality of recovery.
Statistical analysis
Statistical analysis in this study was performed using SPSS 20.0 (IBM Corp., NY, USA). Continuous data were expressed using mean with standard deviation and compared between 2 groups using Student’s t-test. Categorical data were expressed using number with percentage and compared between 2 groups using the chi-square test. Multivariable linear regression analysis was performed to further determine the relationship between the use of esketamine, pain control, and the incidence of PPD after CS. P values less than 0.05 were considered as statistically different.
Results
According to the medical record system of our hospital, 1,138 pregnant women who underwent CS were included in this study and 806 patients were excluded based on the exclusion criteria. Then, 92 out of 332 parturients were excluded because of loss to follow-up. Finally, there were 240 parturients included in this study, with 132 parturients in the control group and 108 parturients in the esketamine group, as shown in Figure 1.
The preoperative data of the included pregnant women are shown in Table 1. The mean age of parturients was 29.6±4.6 years old and the mean pre-pregnancy BMI was 23.6±3.1 kg/m2 in the control group, as shown in Table 1. A total of 107 parturients (81.1%) were full term and more than half of parturients were not primiparas. A total of 63 parturients (47.7%) had a prior history of CS. Most parturients (97.0%) had been married and 33 parturients (25.0%) were unemployed. Fourteen parturients (10.6%) had high pressure during pregnancy and 44 parturients (33.3%) had moderate pressure. Twelve parturients (9.1%) were diagnosed with gestational diabetes and 21 parturients were diagnosed with gestational hypertension. Additionally, 34 parturients (25.8%) were diagnosed with placenta previa. On the other hand, the mean age of parturients was 29.5±4.0 years old and the mean pre-pregnancy BMI was 24.1±3.5 kg/m2 in the esketamine group. There were 41 parturients diagnosed with placenta previa, significantly more than in the control group (P=0.042), and much fewer parturients were unemployed before pregnancy (P=0.019).
Table 1
| Variables | Control group | Esketamine group | P value |
|---|---|---|---|
| Number | 132 | 108 | – |
| Age (year), mean ± SD | 29.6±4.6 | 29.5±4.0 | 0.839 |
| Pre-pregnancy BMI (kg/m2), mean ± SD | 23.6±3.1 | 24.1±3.5 | 0.291 |
| Gestational weeks, n (%) | 0.822 | ||
| Full term | 107 (81.1) | 88 (81.5) | |
| Preterm | 18 (13.6) | 16 (14.8) | |
| Post term | 7 (5.3) | 4 (3.7) | |
| Pregnancy complications, n (%) | |||
| Gestational diabetes | 12 (9.1) | 10 (9.3) | 0.964 |
| Gestational hypertension | 21 (15.9) | 17 (15.7) | 0.972 |
| Acute fatty liver of pregnancy | 8 (6.1) | 7 (6.5) | 0.893 |
| Placenta previa | 34 (25.8) | 41 (38.0) | 0.042 |
| Placental abruption | 4 (3.0) | 5 (4.6) | 0.516 |
| Gravidity, n (%) | 0.667 | ||
| 0 | 50 (37.9) | 38 (35.2) | |
| ≥1 | 82 (62.1) | 70 (64.8) | |
| Parity, n (%) | 0.483 | ||
| 0 | 60 (45.5) | 54 (50.0) | |
| ≥1 | 72 (54.5) | 54 (50.0) | |
| Prior history of CS, n (%) | 63 (47.7) | 52 (48.1) | 0.948 |
| Marital status, n (%) | 0.644 | ||
| Married | 128 (97.0) | 105 (97.2) | |
| Unmarried | 3 (2.3) | 3 (2.8) | |
| Divorced | 1 (0.8) | 0 (0) | |
| Educational level, n (%) | 0.729 | ||
| Master or above | 118 (89.4) | 98 (90.7) | |
| Bachelor or below | 14 (10.6) | 10 (9.3) | |
| Employment, n (%) | 0.019 | ||
| Employed | 99 (75.0) | 94 (87.0) | |
| Unemployed | 33 (25.0) | 14 (13.0) | |
| Pressure during pregnancy, n (%) | 0.742 | ||
| High | 14 (10.6) | 12 (11.1) | |
| Moderate | 44 (33.3) | 31 (28.7) | |
| Low | 74 (56.1) | 65 (60.2) |
SD, standard deviation; BMI, body mass index; CS, cesarean section.
The intraoperative data of the included pregnant women are shown in Table 2, and there was no significant difference between the control group and esketamine group. More than 80% of parturients received elective CS. The mean operation time was 41.5±12.4 minutes in the control group and 43.3±16.5 minutes in the esketamine group. The volume of blood loss was 508.6±285.8 mL in the control group and 520.1±272.0 mL in the esketamine group. Less than 5% of parturients received blood transfusions. The mean dose of esketamine in the esketamine group was 0.35±0.11 mg/kg.
Table 2
| Variables | Control group | Esketamine group | P value |
|---|---|---|---|
| Type of CS, n (%) | 0.245 | ||
| Elective | 109 (82.6) | 95 (88.0) | |
| Emergency | 23 (17.4) | 13 (12.0) | |
| Operation time (minute), mean ± SD | 41.5±12.4 | 43.3±16.5 | 0.041 |
| Anesthesia time (minute), mean ± SD | 75.6±29.1 | 78.2±31.9 | 0.509 |
| Volume of blood loss (mL), mean ± SD | 508.6±285.8 | 520.1±272.0 | 0.752 |
| Blood transfusion, n (%) | 6 (4.5) | 4 (3.7) | 0.745 |
| Fetus, n (%) | 0.588 | ||
| 1 | 121 (91.7) | 101 (93.5) | |
| ≥2 | 11 (8.3) | 7 (6.5) | |
| Dose of esketamine (mg/kg), mean ± SD | – | 0.35±0.11 | – |
CS, cesarean section; SD, standard deviation.
The comparisons of the 2 primary outcomes, namely pain control according to the NRS score and PPD according to the EPDS score, are shown in Figure 2. The NRS scores at rest or when coughing at 2, 4, 8, 24, and 48 hours postpartum were compared between the 2 groups. It was found that all NRS scores at rest at any time point were much lower in the esketamine group than those in the control group. Furthermore, NRS scores when coughing were similar between the 2 groups within 2 hours, and they were much lower in the esketamine group at other time points. There was no significant difference in the EPDS scores at antenatal day 1 between the 2 groups. EPDS scores were much lower in the esketamine group than those in the control group within 3 months postpartum. Due to the retrospective design of this study, the risk factors and predictors of pain control and PPD were then analyzed using multivariable linear regression as shown in Table 3. Emergency CS and longer operation time were identified as risk factors of poor pain control, and esketamine acted as a protector of pain control in these parturients who underwent CS. Similarly, esketamine was confirmed to improve the incidence of PPD, and primiparas, high pressure during pregnancy, and high NRS score within 48 hours contributed to the increase of PPD.
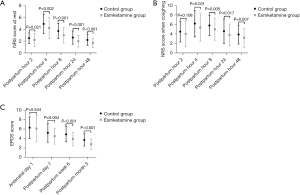
Table 3
| Characteristics | Multivariable analysis | ||
|---|---|---|---|
| β | 95% CI | P value | |
| Pain control based on NRS score | |||
| Esketamine | −0.687 | −0.962, −0.412 | <0.001 |
| Emergency CS | 0.793 | 0.413, 1.174 | <0.001 |
| Longer operation time | 0.024 | 0.001, 0.046 | 0.039 |
| PPD based on EPDS score | |||
| Esketamine | −0.404 | −0.655, −0.153 | 0.002 |
| Primiparas | 0.250 | 0.006, 0.495 | 0.045 |
| High pressure during pregnancy | 0.716 | 0.539, 0.894 | <0.001 |
| High NRS score within 48 hours | 0.144 | 0.064, 0.224 | 0.023 |
PPD, postpartum depression; CI, confidence interval; NRS, numeric rating scale; CS, cesarean section; EPDS, Edinburgh postnatal depression scale.
Secondary outcomes are also shown in Table 4. The incidence of analgesia-related adverse events was slightly higher in the esketamine group than that in the control group, showing no significant difference. Parturients in the esketamine group had dramatically better sleep quality within 1 week postpartum (P=0.044). In addition, morphine consumption within 24 hours postpartum was lower in the esketamine group than in the control group (P<0.001). The GAD-7 score, indicating postpartum anxiety within 1 week postpartum, was lower in the esketamine group (P<0.001), and the quality of recovery within 3 months postpartum according to the QoR-15 score was also better in the esketamine group (P=0.001).
Table 4
| Variables | Control group | Esketamine group | P value |
|---|---|---|---|
| Analgesia-related adverse events, n (%) | |||
| Nausea and vomiting | 26 (19.7) | 27 (25.0) | 0.324 |
| Dizziness | 37 (28.0) | 42 (38.9) | 0.075 |
| Pruritus | 9 (6.8) | 11 (10.2) | 0.348 |
| Sleep quality within 1 week postpartum, n (%) | 0.044 | ||
| Good | 24 (18.2) | 31 (28.7) | |
| Moderate | 30 (22.7) | 30 (27.8) | |
| Poor | 78 (59.1) | 47 (43.5) | |
| Morphine consumption within 24 hours postpartum (mg), mean ± SD | 32.3±12.3 | 24.1±11.3 | <0.001 |
| Postpartum anxiety within 1 week postpartum, mean ± SD | 7.2±2.1 | 6.2±2.2 | <0.001 |
| Quality of recovery within 3 months postpartum, mean ± SD | 114.8±19.0 | 124.7±25.9 | 0.001 |
SD, standard deviation.
A subgroup analysis was then performed and parturients in the esketamine group were divided into 2 subgroups according to the dose of esketamine. Comparing the perioperative data of included parturients as shown in Table 5, the mean pre-pregnancy BMI was much lower in the high-dose group than that in the low-dose group, and the mean dose of esketamine was 0.25±0.05 mg/kg in the low-dose group, which was lower than 0.42±0.08 mg/kg in the high-dose group. No significant difference was found in other data between the 2 subgroups. NRS scores and EPDS scores in the 2 subgroups are shown in Figure 3. NRS scores at rest at 24 and 48 hours postpartum in the high-dose group were much lower than those in the low-dose group (P<0.001 and P=0.011, respectively). Also, the NRS score when coughing at 24 hours postpartum was lower in the high-dose group compared with the low-dose group. However, no significant difference was found between the 2 subgroups in postpartum EPDS scores. Secondary outcomes of the 2 subgroups were compared as shown in Table 6. The incidence of analgesia-related adverse events was slightly higher in the high-dose group, however, there was no significant difference compared with the low-dose group. Similarly, no significant difference was found between the 2 subgroups in other secondary outcomes.
Table 5
| Variables | Low-dose group | High-dose group | P value |
|---|---|---|---|
| Number | 40 | 68 | – |
| Age (year), mean ± SD | 28.9±3.2 | 29.8±4.4 | 0.248 |
| Pre-pregnancy BMI (kg/m2), mean ± SD | 26.7±1.7 | 23.0±2.4 | <0.001 |
| Gestational weeks, n (%) | 0.056 | ||
| Full term | 28 (70.0) | 60 (88.2) | |
| Preterm | 10 (25.0) | 6 (8.8) | |
| Post term | 2 (5.0) | 2 (2.9) | |
| Pregnancy complications, n (%) | |||
| Gestational diabetes | 5 (12.5) | 5 (7.4) | 0.373 |
| Gestational hypertension | 4 (10.0) | 13 (19.1) | 0.209 |
| Acute fatty liver of pregnancy | 2 (5.0) | 5 (7.4) | 0.483 |
| Placenta previa | 12 (30.0) | 29 (42.6) | 0.191 |
| Placental abruption | 1 (2.5) | 4 (5.9) | 0.386 |
| Gravidity, n (%) | 0.101 | ||
| 0 | 18 (45.0) | 20 (29.4) | |
| ≥1 | 22 (55.0) | 48 (70.6) | |
| Parity, n (%) | 1.000 | ||
| 0 | 20 (50.0) | 34 (50.0) | |
| ≥1 | 20 (50.0) | 34 (50.0) | |
| Prior history of CS, n (%) | 16 (40.0) | 36 (52.9) | 0.194 |
| Marital status, n (%) | 1.000 | ||
| Married | 39 (97.5) | 66 (97.1) | |
| Unmarried | 1 (2.5) | 2 (2.9) | |
| Divorced | 0 (0) | 0 (0) | |
| Educational level, n (%) | 0.373 | ||
| Master or above | 35 (87.5) | 63 (92.6) | |
| Bachelor or below | 5 (12.5) | 5 (7.4) | |
| Employment, n (%) | 0.913 | ||
| Employed | 35 (87.5) | 59 (86.8) | |
| Unemployed | 5 (12.5) | 9 (13.2) | |
| Pressure during pregnancy, n (%) | 0.405 | ||
| High | 6 (15.0) | 6 (8.8) | |
| Moderate | 13 (32.5) | 18 (26.5) | |
| Low | 21 (52.5) | 44 (64.7) | |
| Type of CS, n (%) | 0.364 | ||
| Elective | 37 (92.5) | 58 (85.3) | |
| Emergency | 3 (7.5) | 10 (14.7) | |
| Operation time (minute), mean ± SD | 44.5±15.0 | 42.7±17.4 | 0.581 |
| Anesthesia time (minute), mean ± SD | 78.5±33.9 | 78.0±30.9 | 0.936 |
| Volume of blood loss (mL), mean ± SD | 541.0±300.3 | 507.9±255.5 | 0.544 |
| Blood transfusion, n (%) | 2 (5.0) | 2 (2.9) | 0.626 |
| Fetus, n (%) | 0.708 | ||
| 1 | 37 (92.5) | 64 (94.1) | |
| ≥2 | 3 (7.5) | 4 (5.9) | |
| Dose of esketamine (mg/kg), mean ± SD | 0.25±0.05 | 0.42±0.08 | <0.001 |
BMI, body mass index, CS, cesarean section; SD, standard deviation.
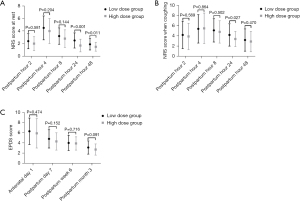
Table 6
| Variables | Low-dose group | High-dose group | P value |
|---|---|---|---|
| Analgesia-related adverse events, n (%) | |||
| Nausea and vomiting | 7 (17.5) | 20 (29.4) | 0.167 |
| Dizziness | 14 (35.0) | 28 (41.1) | 0.525 |
| Pruritus | 3 (7.5) | 8 (11.8) | 0.743 |
| Sleep quality within 1 week postpartum, n (%) | 0.968 | ||
| Good | 11 (27.5) | 20 (29.4) | |
| Moderate | 11 (27.5) | 19 (27.9) | |
| Poor | 18 (45.0) | 29 (42.6) | |
| Morphine consumption within 24 hours postpartum (mg), mean ± SD | 23.7±10.9 | 24.3±11.6 | 0.787 |
| Postpartum anxiety within 1 week postpartum, mean ± SD | 6.1±2.1 | 6.3±2.3 | 0.754 |
| Quality of recovery within 3 months postpartum, mean ± SD | 122.2±26.2 | 126.5±25.8 | 0.254 |
SD, standard deviation.
Discussion
Very few studies have been performed to investigate the effects of esketamine on pain control and the incidence of PPD in pregnant women who have undergone CS. This study retrospectively collected some perioperative data and analyzed data using multivariable linear regression, which found that the use of esketamine was beneficial for both pain control and improving PPD. It was also found that the effects of low-dose esketamine on the prognosis of parturients were similar to those of high-dose esketamine.
Many previous studies have investigated the effects of other potential drugs on pain control or the incidence of PPD. Dexmedetomidine was reported by Yu et al. to be useful for alleviating PPD following CS (18). Tramadol was also reported by Wu et al. to ameliorate PPD in high-risk woman after CS (12). On the other hand, inhalation of chamomile oil was reported to relieve CS pain in primiparous women (19). Shahraki et al. also reported that both oral methadone and intramuscular pethidine provided similar analgesic effects after CS (20). However, most of the above drugs can only relieve postoperative pain or reduce depressive symptoms alone, but drugs that can relieve both pain and PPD are rare. Esketamine is an NMDA receptor agonist and has a higher affinity for NMDA receptors than ketamine. NMDA receptor subunits have complex physiological functions, which can regulate the survival of neurons, the development of dendrites and axons and synaptic plasticity, and affect the formation of neurons and the process of learning and memory (21,22). As an agonist of NMDA receptors, esketamine was reported to be useful for the treatment of depressive disorder (23,24). Furthermore, esketamine was confirmed to be useful for pain control in surgical patients (15,16). Suppa et al. reported in 2012 that low-dose esketamine could effectively control postoperative pain in 56 pregnant women who underwent CS (25). Our study further reported the beneficial effects of esketamine on pain control and the incidence of PPD in pregnant women who underwent CS. Also, the consumption of morphine could be reduced by the use of esketamine, which was similar to a previous study (15).
Some other risk factors of poor pain control and PPD were also identified in this study. Emergency CS and longer operation time were related to poor pain control, while primiparas, high pressure during pregnancy, and high NRS score within 48 hours contributed to the incidence of PPD. Shen et al. reported previously that primiparas, emergency surgery, and NRS score of more than 1 point were related to the incidence of PPD in parturients who underwent CS (8). Yu et al. reported that domestic violence and life stress events also contributed to the incidence of PPD (18). However, the data of domestic violence and life stress events could not be obtained due to the retrospective design of this study.
The dose of esketamine used in parturients has not yet been determined. Generally, the dose of esketamine is 0.25 or 0.5 mg/kg (25,26). In cervical carcinoma patients, both 0.5 and 0.25 mg/kg esketamine improved short-term depression and pain after surgery, showing no difference between the 2 groups (27). In contrast, esketamine was reported to reduce the consumption of an opioid in a dose-dependent manner in patients who underwent major lumbar fusion surgery (28). Our study found that NRS scores at rest at 24 and 48 hours postpartum in the high-dose group were much lower than those in the low-dose group (P<0.001 and P=0.011, respectively). Also, the NRS score when coughing at 24 hours postpartum was lower in the high-dose group compared with the low-dose group. No significant difference was found between the 2 subgroups in postpartum EPDS scores. However, the incidence of analgesia-related adverse events was slightly higher in the high-dose group. Therefore, low-dose esketamine may be more suitable for pregnant women undergoing CS, considering the potential adverse events and similar effects to high-dose esketamine.
There were some limitations in this study. Firstly, our center has used esketamine for analgesia after CS since 2019. There was a certain time difference between the control group and the esketamine group. This may have led to certain differences in the medical level and parturient prognosis. Secondly, the dose of esketamine used in this study was 20 mg, thus, the different doses in the subgroup analysis were mainly dependent on the different body weights of pregnant women. This may have led to selection bias in this study. A randomized controlled study will be more helpful to verify the effect of different doses of esketamine on the prognosis of parturients. Thirdly, this study was a retrospective study, which may have contributed to some errors in the collected data. Also, some data, such as spousal relationship, family income, and domestic violence, could not be obtained in this study.
Conclusions
This retrospective study confirmed the effects of esketamine on pain control and the incidence of PPD in pregnant women who underwent CS. Furthermore, the use of esketamine could reduce the consumption of morphine and improve the quality of recovery. The subgroup analysis revealed that low-dose and high-dose esketamine provided similar improvement of postoperative pain and PPD. Considering the potential adverse events induced by esketamine, low-dose esketamine may be more suitable for pregnant women who have undergone CS.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-21-3343/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-21-3343/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-21-3343/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Wuxi No. 9 People’s Hospital Affiliated to Soochow University (No. 0021022). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhang J, Zhang Y, Ma Y, et al. The associated factors of cesarean section during COVID-19 pandemic: a cross-sectional study in nine cities of China. Environ Health Prev Med 2020;25:60. [Crossref] [PubMed]
- Su Y, Heitner J, Yuan C, et al. Effect of a Text Messaging-Based Educational Intervention on Cesarean Section Rates Among Pregnant Women in China: Quasirandomized Controlled Trial. JMIR Mhealth Uhealth 2020;8:e19953. [Crossref] [PubMed]
- Li L, Cui H. The risk factors and care measures of surgical site infection after cesarean section in China: a retrospective analysis. BMC Surg 2021;21:248. [Crossref] [PubMed]
- Huang L, Zhang J, Sun H, et al. Association of gestational weight gain with cesarean section: a prospective birth cohort study in Southwest China. BMC Pregnancy Childbirth 2021;21:57. [Crossref] [PubMed]
- Sharma LR, Schaldemose EL, Alaverdyan H, et al. Perioperative factors associated with persistent post-surgical pain after hysterectomy, cesarean section, prostatectomy, and donor nephrectomy: a systematic review and meta-analysis. Pain 2021; [Epub ahead of print]. [Crossref] [PubMed]
- Cao X, Zhang X. Comparison of different sufentanil-tramadol combinations for pain relief within the first 24 hours after cesarean section: a retrospective study. J Pain Res 2018;11:2445-51. [Crossref] [PubMed]
- Mäkelä K, Palomäki O, Pokkinen S, et al. Oral versus patient-controlled intravenous administration of oxycodone for pain relief after cesarean section. Arch Gynecol Obstet 2019;300:903-9. [Crossref] [PubMed]
- Shen D, Hasegawa-Moriyama M, Ishida K, et al. Acute postoperative pain is correlated with the early onset of postpartum depression after cesarean section: a retrospective cohort study. J Anesth 2020;34:607-12. [Crossref] [PubMed]
- Chandrasekaran N, De Souza LR, Urquia ML, et al. Is anemia an independent risk factor for postpartum depression in women who have a cesarean section? - A prospective observational study. BMC Pregnancy Childbirth 2018;18:400. [Crossref] [PubMed]
- Xu H, Ding Y, Ma Y, et al. Cesarean section and risk of postpartum depression: A meta-analysis. J Psychosom Res 2017;97:118-26. [Crossref] [PubMed]
- Wang SY, Duan KM, Tan XF, et al. Genetic variants of the kynurenine-3-monooxygenase and postpartum depressive symptoms after cesarean section in Chinese women. J Affect Disord 2017;215:94-101. [Crossref] [PubMed]
- Wu Z, Zhao P, Peng J, et al. A Patient-Controlled Intravenous Analgesia With Tramadol Ameliorates Postpartum Depression in High-Risk Woman After Cesarean Section: A Randomized Controlled Trial. Front Med (Lausanne) 2021;8:679159. [Crossref] [PubMed]
- Brinck ECV, Maisniemi K, Kankare J, et al. Analgesic Effect of Intraoperative Intravenous S-Ketamine in Opioid-Naïve Patients After Major Lumbar Fusion Surgery Is Temporary and Not Dose-Dependent: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Anesth Analg 2021;132:69-79. [Crossref] [PubMed]
- Ng J, Rosenblat JD, Lui LMW, et al. Efficacy of ketamine and esketamine on functional outcomes in treatment-resistant depression: A systematic review. J Affect Disord 2021;293:285-94. [Crossref] [PubMed]
- Nielsen RV, Fomsgaard JS, Nikolajsen L, et al. Intraoperative S-ketamine for the reduction of opioid consumption and pain one year after spine surgery: A randomized clinical trial of opioid-dependent patients. Eur J Pain 2019;23:455-60. [Crossref] [PubMed]
- Liu P, Li P, Li Q, et al. Effect of Pretreatment of S-Ketamine On Postoperative Depression for Breast Cancer Patients. J Invest Surg 2021;34:883-8. [Crossref] [PubMed]
- Xie M, Lao TT, Du M, et al. Risk for Cesarean section in women of advanced maternal age under the changed reproductive policy in China: A cohort study in a tertiary hospital in southwestern China. J Obstet Gynaecol Res 2019;45:1866-75. [Crossref] [PubMed]
- Yu HY, Wang SY, Quan CX, et al. Dexmedetomidine Alleviates Postpartum Depressive Symptoms following Cesarean Section in Chinese Women: A Randomized Placebo-Controlled Study. Pharmacotherapy 2019;39:994-1004. [Crossref] [PubMed]
- Zardosht R, Basiri A, Sahebkar A, et al. Effect of Chamomile Oil on Cesarean Section Pain in Primiparous Women: A Randomized Clinical Trial. Curr Rev Clin Exp Pharmacol 2021;16:369-74. [Crossref] [PubMed]
- Shahraki AD, Jabalameli M, Ghaedi S. Pain relief after cesarean section: Oral methadone vs. intramuscular pethidine. J Res Med Sci 2012;17:143-7. [PubMed]
- Treccani G, Ardalan M, Chen F, et al. S-Ketamine Reverses Hippocampal Dendritic Spine Deficits in Flinders Sensitive Line Rats Within 1 h of Administration. Mol Neurobiol 2019;56:7368-79. [Crossref] [PubMed]
- Salahudeen MS, Wright CM, Peterson GM. Esketamine: new hope for the treatment of treatment-resistant depression? A narrative review. Ther Adv Drug Saf 2020;11:2042098620937899. [Crossref] [PubMed]
- Zheng W, Cai DB, Xiang YQ, et al. Adjunctive intranasal esketamine for major depressive disorder: A systematic review of randomized double-blind controlled-placebo studies. J Affect Disord 2020;265:63-70. [Crossref] [PubMed]
- Wang SM, Kim NY, Na HR, et al. Rapid Onset of Intranasal Esketamine in Patients with Treatment Resistant Depression and Major Depression with Suicide Ideation: A Meta-Analysis Clin Psychopharmacol Neurosci 2021;19:341-54. [Crossref] [PubMed]
- Suppa E, Valente A, Catarci S, et al. A study of low-dose S-ketamine infusion as "preventive" pain treatment for cesarean section with spinal anesthesia: benefits and side effects. Minerva Anestesiol 2012;78:774-81. [PubMed]
- Kadic L, van Haren FG, Wilder-Smith O, et al. The effect of pregabalin and s-ketamine in total knee arthroplasty patients: A randomized trial. J Anaesthesiol Clin Pharmacol 2016;32:476-82. [Crossref] [PubMed]
- Wang J, Wang Y, Xu X, et al. Use of Various Doses of S-Ketamine in Treatment of Depression and Pain in Cervical Carcinoma Patients with Mild/Moderate Depression After Laparoscopic Total Hysterectomy. Med Sci Monit 2020;26:e922028. [Crossref] [PubMed]
- Brinck ECV, Virtanen T, Mäkelä S, et al. S-ketamine in patient-controlled analgesia reduces opioid consumption in a dose-dependent manner after major lumbar fusion surgery: A randomized, double-blind, placebo-controlled clinical trial. PLoS One 2021;16:e0252626. [Crossref] [PubMed]
(English Language Editor: C. Betlzar)



