
Clinical experience of transbronchoscopic laser ablation for central airway stenosis using a high-power diode laser—ten years’ experience at a single institute
Introduction
Airway stenosis is a medical emergency, and interventions to maintain airway patency are required, regardless of the cause of stenosis (1). High-power laser ablation has an advantage over other argon plasma coagulation (APC), as it can reopen the airway stenosis quickly via laser vaporization. For airway stenosis due to a malignant tumor, a high-power laser is often used in combination with the insertion of several stents. A high-power laser is also used to treat airway stenosis due to tuberculosis scars and post-intubation granulation (2). Regarding high-power laser systems, an Nd-YAG laser was the most common instrument in endobronchial therapy; however, the instrument was quite large and expensive. The newly developed high-power diode (GaAlAs) laser system is more compact and easier to handle than a conventional Nd-YAG laser. APC was also used during endoscopic therapy as an alternative to high-power laser ablation (3); however, APC is based on high-frequency cauterization, mainly heat coagulation and heat transpiration, which require a longer time to achieve recanalization of the airway. Because of the several advantages associated with a high-power diode (GaAlAs) laser system over a conventional Nd-YAG laser, we often use high-power diode (GaAlAs) laser ablation in interventional pulmonology. To deliver the clinical value of diode laser systems, we retrospectively reviewed the patients treated for central airway lesions by laser ablation using a high-power diode laser system. We herein review our experience in performing transbronchoscopic laser ablation to explore a better clinical approach for managing both neoplastic and non-neoplastic central airway lesions. We present the following article in accordance with the STROBE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-21-2273/rc).
Methods
We performed a retrospective chart review of the patients who were treated for central airway lesions using a transbronchial laser ablation system with a non-contact-type probe between January 2005 and December 2015 at Chiba University Hospital. All patients were reviewed for the cause of stenosis, number of interventions, laser setting, total amount of laser energy application, complications, and simultaneously performed modalities. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethical review board of Chiba University Graduate School of Medicine (No. 1139) and individual consent for this retrospective study was waived.
A semiconductor diode laser (UDL-60; Olympus, Tokyo, Japan) with a non-contact laser probe was used for transbronchoscopic laser ablation. The procedure was performed through a video bronchoscope with a large instrument channel (internal diameter: 2.6 mm). Laser ablation was performed with an output of 15–35 W, 1.0-second pulsed mode. Patients was given local anesthesia via inhalation as well as sprayed lidocaine and intravenous conscious sedative medication using midazolam; in addition, they were intubated before the start of intervention, and the oxygen supply was intermittently suspended during the ablative therapy. The critical point was to keep the oxygen concentration under 40% during intervention.
Statistical analysis
The data analysis was performed following standard definitions.
Results
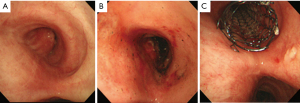
Thirty-three patients were included in this analysis, and the patients underwent intervention a total of 72 times. There were 23 males and 10 females with a median age of 60.3 years old (range, 18–80 years old). The primary causes of the central airway stenosis were neoplastic disease in 22 (16 malignant tumors, 6 benign tumors) and non-neoplastic disease in 11 (Table 1). Among malignant tumors, there were 13 lung cancers, including 8 tracheal and 3 esophageal. Among benign tumors, there were three hamartomas and one patient each with papilloma, smooth muscle tumor, and glomus tumor (Table 1). The non-neoplastic causes of airway stenosis were intubation or tracheotomy-related stricture in four; tuberculosis, inflammatory granuloma, and trauma/burn in two each; and one surgical complication after bronchoplasty (Table 2). The number of treatment sessions per patient was 3.82 times on average for non-neoplastic causes of airway stenosis, which was more frequent than for those with neoplastic causes (1.36 sessions on average; P<0.05) (Tables 1,2). The total amount of laser energy applied was 1,936 J on average (1,674 J for neoplastic disease and 2,098 J for non-neoplastic disease). More laser energy was applied for non-neoplastic disease than for neoplastic disease (P<0.01). Regarding the simultaneously performed interventions, 18 of 22 patients were treated with a combination of snaring, ethanol injection, and stent insertion for neoplastic diseases. For non-neoplastic disease, 4 out of 11 patients were treated with a combination of balloon dilatation and snaring. Three cases with malignant stenosis received a bronchial stent. No cases received an esophageal stent. We have now included photographic documentation showing the effect of the treatment in Figure 1. No patients underwent other forms of laser ablation during this period. No cases required conversion to rigid bronchoscopy. The median duration of follow-up was 741 [4–4,741] days. Success was defined as the restoration of the trachea. Restoration of the trachea was achieved in all cases temporarily. The major complication of this treatment was re-stenosis of the trachea, which occurred in six cases of non-neoplastic diseases and three of malignant diseases. We performed laser ablation repeatedly in these cases. Five cases of non-neoplastic diseases did not need any further interventions. There were no major complications related to endoscopic laser ablation therapy other than re-stenosis of the trachea. Surgery for benign stenosis was not performed because of the patient’s wish. Many patients are able to keep their trachea open with intervention alone and do not wish to undergo surgery. The mortality in this series was 7 cases (43.8%) in the malignant group and 2 cases in the non-malignant group during the follow-up period. The cause of death in the non-malignant group was another illness. The reasons of not to undergo surgery were patient’s wishes. Many patients could keep trachea opened by intervention only and did not want to have surgery. There was one patient requiring surgery in the non-malignant tumor group after laser ablation. In that case, we performed snaring to remove the tumor and used laser ablation to stop the bleeding. The tumor was not entirely removed by snaring, so we performed S6 segmentectomy.
Table 1
| Causes of airway stenosis | Number of patients | Number of treatments per patient (average) |
|---|---|---|
| Bronchial cancer | 8 | 1.4 |
| Lung cancer | 5 | 1.2 |
| Esophageal cancer | 3 | 1.3 |
| Hamartoma | 3 | 1.3 |
| Papilloma | 1 | 2 |
| Smooth muscle tumor | 1 | 1 |
| Glomus tumor | 1 | 2 |
| Total | 22 | 1.4 |
Table 2
| Causes of airway stenosis | Number of patients | Number of treatments per patient (average) |
|---|---|---|
| Intubation or tracheotomy | 4 | 3.8 |
| Tuberculosis | 2 | 5 |
| Granulation | 2 | 1 |
| Trauma or burn | 2 | 5 |
| Surgery | 1 | 2 |
| Total | 11 | 3.8 |
Discussion
High-power laser ablation is often used for managing central airway lesions, regardless of the etiology, especially for patients with symptoms associated with central airway stenosis. One advantage of applying high-power laser ablation for central airway stenosis is its laser vaporization effect, allowing the airway to be reopened quickly. A high-power laser system has also been used to achieve hemostasis for bleeding from endobronchial lesions. We often use laser ablation during interventions, especially for easily bleeding tumors due, to the photocoagulation effect (4,5). Pre-interventional assessments are very important for high-power laser ablation because this approach cannot be used for central airway stenosis due to extraluminal compression or lesions adjacent to the peripheral respiratory tract (4). Patients with tracheoesophageal fistula are also contraindicated for laser ablation. Essential knowledge that the high-power laser reaches deeper than expected from the visual change of the surface. Unexpected tissue damage sometimes causes serious complications, such as tracheal perforation or massive bleeding. In particular, Nd-YAG lasers can reach 3–5 mm below the surface, causing more serious complications (4-6) than a high-power diode (GaAlAs) laser (7). High-power laser treatment was able to be performed under local anesthesia with moderate sedation through endotracheal intubation. Systematic monitoring of the blood pressure, heart rate, and respiratory condition, including SpO2 tracking, was necessary. The most critical point to keep in mind when operators perform laser ablation is that the oxygen concentration must be kept under 40% to prevent fire break-out and burn injury within the respiratory tract (5,7). In conclusion, transbronchoscopic laser ablation using a diode laser system with a non-contact probe can be safely performed and is useful for endobronchial treatment of both neoplastic and non-neoplastic central airway lesions.
Acknowledgments
We thank Brian Quinn from Japan Medical Communication for editing a draft of this manuscript.
Funding: This work was supported by JSPS KAKENHI (grant No. 21K08880).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-21-2273/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-21-2273/dss
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-21-2273/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-21-2273/coif). TN received honoraria and lecture fees from Olympus and AstraZeneca for continuous medical education. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethical review board of Chiba University Graduate School of Medicine (No. 1139) and individual consent for this retrospective study was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ernst A, Silvestri GA, Johnstone D, et al. Interventional pulmonary procedures: Guidelines from the American College of Chest Physicians. Chest 2003;123:1693-717. [Crossref] [PubMed]
- Bolliger CT, Sutedja TG, Strausz J, et al. Therapeutic bronchoscopy with immediate effect: laser, electrocautery, argon plasma coagulation and stents. Eur Respir J 2006;27:1258-71. [Crossref] [PubMed]
- Wahidi MM, Herth FJ, Ernst A. State of the art: interventional pulmonology. Chest 2007;131:261-74. [Crossref] [PubMed]
- Shulimzon TR. Interventional pulmonology: a new medical specialty. Isr Med Assoc J 2014;16:379-84. [PubMed]
- Shepherd RW, Radchenko C. Bronchoscopic ablation techniques in the management of lung cancer. Ann Transl Med 2019;7:362. [Crossref] [PubMed]
- Fisher JC. The power density of a surgical laser beam: its meaning and measurement. Lasers Surg Med 1983;2:301-15. [Crossref] [PubMed]
- Guibert N, Mhanna L, Droneau S, et al. Techniques of endoscopic airway tumor treatment. J Thorac Dis 2016;8:3343-60. [Crossref] [PubMed]


