
The efficacy of pyrotinib-based therapy in lapatinib-resistant metastatic HER2-positive breast cancer
Introduction
Human epidermal growth factor receptor 2 (HER2) positivity and HER2 gene amplification account for approximately 20% of all breast cancers (1). HER2-positive breast cancer tends to metastasize and is associated with poor prognosis. The overall survival (OS) of HER2-positive breast cancer has been prolonged since the use of anti-HER2 targeted therapy. Chemotherapy and dual-blockade HER2 targeted therapy with trastuzumab and pertuzumab is the standard first-line treatment for HER2-positive metastatic breast cancer (MBC) (2) due to its progression-free survival (PFS) and OS benefits. For the second-line treatment of HER2-positive MBC, ado-trastuzumab emtansine (T-DM1)-containing therapies are candidate regimens (3). T-DM1 is approved for MBC in China, but it’s not covered by medical insurance. Lapatinib, a tyrosine kinase inhibitor (TKI) targeting epidermal growth factor receptor and HER2, combined with chemotherapy is the recommended second-line anti-HER2 treatment in China (3). For later lines of therapy in HER2-positive MBC, there is no standard treatment. Anti-HER2 targeted therapy combined with chemotherapy, endocrine therapy, or another targeted therapy is usually used. Pyrotinib is a new TKI which could block epidermal growth factor receptor, HER2, and HER4. Thirty-eight patients were enrolled in the phase 1 study, who received in the 80- to 400-mg dose cohorts. The dose-limiting toxicity was grade 3 diarrhea, and the maximum tolerated dose was 400 mg. Pyrotinib was approved for HER2-positive MBC in mainland China in late 2018 based on its excellent efficacy, showing prolonged PFS and increased objective response rate (ORR) in a phase II study (4,5). A randomized, double-blind, placebo-controlled phase III study (PHENIX) showed that pyrotinib plus capecitabine had a significant increase in PFS for HER2-positive MBC after prior trastuzumab and taxanes (6). The PHOEBE study demonstrated that pyrotinib plus capecitabine had prolonged PFS compared with lapatinib and capecitabine in trastuzumab-treated TKI-untreated patients (7). Pyrotinib could block one more pathway, HER4, compared with lapatinib in terms of mechanism. However, the efficacy of pyrotinib in lapatinib-resistant patients is not reported in previous studies. Since pyrotinib can block the HER4 pathway, which cannot be blocked by lapatinib, pyrotinib may be effective in lapatinib-resistant MBC patients. This study aimed to evaluate the effects of pyrotinib-containing treatment in lapatinib-resistant patients in the third or later line settings. We present the following article in accordance with the STROBE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-21-3965/rc).
Methods
Patients
This retrospective observational cohort study enrolled MBC patients from Ruijin Hospital Shanghai Jiaotong University School of Medicine in China between August 1, 2018 and September 30, 2020. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Ruijin Hospital Shanghai Jiaotong University School of Medicine (Ethic Committee Reference Number 2021178). As this retrospective study did not harm the rights and health of patients, and protected their privacy and personal information, the ethics committee waived the requirement to obtain informed consent. Participants were female patients with HER2-positive MBC receiving pyrotinib-containing therapy in Ruijin Hospital. The inclusion criteria were as follows: (I) patients with histologically confirmed HER2-positive MBC (3+ staining intensity by immunohistochemical analysis and/or HER2 gene amplification by fluorescence in situ hybridization); (II) patients with adequate hematological, hepatic, and renal function; (III) prior disease progression during treatment with lapatinib; (IV) at least 1 measureable lesion according to the Response Evaluation Criteria in Solid Tumors guidelines (RECIST version 1.1). Routine clinical information was documented and collected from an electronic case record system by two physicians.
Treatment and dose adjustment
Patients were received the target treatment of pyrotinib (400 mg orally once daily). Combination treatment with cytotoxic drugs, anti-HER2 drugs, or endocrine therapy drugs was determined according to patients’ physical status and prior regimens used. Dose adjustment, dose interruption, and treatment discontinuation were decided by the physician according to the side-effects.
Outcome and safety assessments
Clinical follow-up was conducted weekly and radiographic examinations were conducted every 3 cycles of treatment (for pyrotinib combined with endocrine therapy, radiographic examinations were performed every 2 months). Tumor response assessments were made according to RECIST criteria (version 1.1) using radiological scans, including computed tomography (CT) or magnetic resonance imaging (MRI). Adverse events (AEs) were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE, 4.0). AEs were collected from a patient self-reporting system and laboratory test results.
The primary endpoint was ORR, which was defined as the proportion of patients with complete response (CR) or partial response (PR). Secondary endpoints included PFS, clinical benefit rate (CBR), OS, and safety. PFS was defined as the time from starting pyrotinib treatment to the date of disease progression confirmed by CT/MRI scan or death of any cause, whichever occurred first. CBR was defined as the proportion of patients with CR, PR, and stable disease (SD). OS was defined as the time period from starting pyrotinib treatment to the date of death of any cause.
Statistical analysis
Median PFS (mPFS) were calculated by the Kaplan-Meier method and subgroup comparisons were evaluated by the log-rank test. The median follow-up time was calculated by the reverse Kaplan-Meier method. The stepwise Cox regression model was used to analyze the correlations between factors and PFS. All statistical analyses were performed using SPSS version 19 (SPSS Inc., Chicago, IL, USA). All statistical tests were two-tailed and P<0.05 was considered statistically significant.
Results
Baseline characteristics
Between August 1, 2018 and September 30, 2020, a total of 31 patients were enrolled in this study. Among these patients, the mean age was 55.9 (range, 31 to 69) years. The baseline characteristics are summarized in Table 1. Six patients (19.4%) had brain metastasis. All patients were heavily treated MBC, and they all had prior trastuzumab-containing therapy, with 32.3% of patients treated in the adjuvant or neoadjuvant setting (2 patients received neoadjuvant treatment), 15% of patients treated in the palliative setting, and the remaining 19.4% of patients treated in both the adjuvant and palliative settings. All patients had prior lapatinib-containing therapy. One patient had prior pertuzumab-containing therapy, and 2 patients had prior T-DM1 treatment. The median number of previous lines of anti-HER2 treatment was 3 (range, 2–7) lines. Twenty-nine of the 31 patients (93.5%) concurrently received cytotoxic drugs. Of these, 12 patients (38.7%) received vinorelbine, 8 patients (25.8%) received capecitabine, 4 patients (12.9%) received paclitaxel, 3 patients (9.7%) received gemcitabine, and 2 patients (6.5%) received nab-paclitaxel. One patient concurrently received letrozole, and another patient concurrently received trastuzumab.
Table 1
| Variables | N | % |
|---|---|---|
| Age | ||
| Mean ± standard deviation | 55.9±10.4 | |
| Menopausal status | ||
| Premenopausal | 8 | 74.2 |
| Postmenopausal | 23 | 25.8 |
| ECOG PS | ||
| 0–1 | 21 | 67.7 |
| 2–3 | 10 | 32.3 |
| ER | ||
| Positive | 9 | 29.0 |
| Negative | 22 | 71.0 |
| De novo stage IV | ||
| Yes | 5 | 16.1 |
| No | 26 | 83.9 |
| Visceral metastasis | ||
| Yes | 23 | 74.2 |
| No | 8 | 25.8 |
| Liver metastasis | ||
| Yes | 9 | 29.0 |
| No | 22 | 71.0 |
| Brain metastasis | ||
| Yes | 6 | 19.4 |
| No | 25 | 80.6 |
| Trastuzumab use | ||
| Adjuvant or neoadjuvant only | 10 | 32.3 |
| Palliative only | 15 | 48.4 |
| Adjuvant and palliative | 6 | 19.4 |
| Pertuzumab use | ||
| Yes | 1 | 3.2 |
| No | 30 | 96.8 |
| T-DM1 use | ||
| Yes | 2 | 6.5 |
| No | 29 | 93.5 |
| Lapatinib use | ||
| Yes | 31 | 100.0 |
| No | 0 | 0.0 |
| Previous lines of palliative treatment | ||
| Median [range] | 3 [2–7] | |
| Combined treatment agent | ||
| Vinorelbine | 12 | 38.7 |
| Capecitabine | 8 | 25.8 |
| Paclitaxel | 4 | 12.9 |
| Gemcitabine | 3 | 9.7 |
| Nab-paclitaxel | 2 | 6.5 |
| Letrozole | 1 | 3.2 |
| Trastuzumab | 1 | 3.2 |
ECOG PS, Eastern Cooperative Oncology Group performance status; ER, estrogen receptor; T-DM1, ado-trastuzumab emtansine.
Efficacy
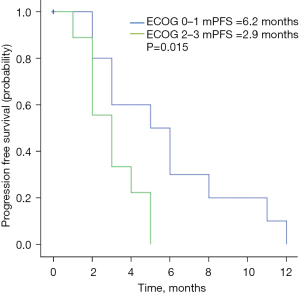
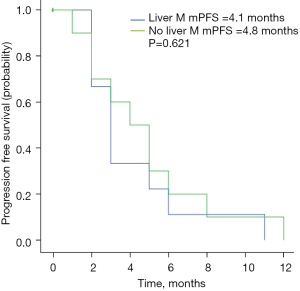
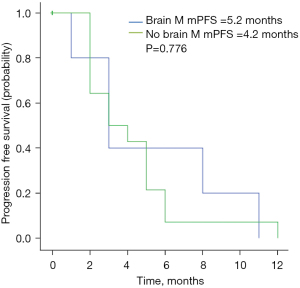
As of November 2020, the median follow-up duration was 11.4 months. The radiological response at the first assessment is shown in Table 2. No patient achieved CR, while 8 patients (25.8%) achieved PR. The ORR was 25.8%. Thirteen patients (41.9%) achieved SD for a CBR of 67.7%. The mPFS in the study population was 4.5 months (95% CI: 3.1–5.9 months; Figure 1). Twelve patients were still in treatment and the mOS was not achieved by the time of this study. Physical status [Eastern Cooperative Oncology Group (ECOG) 0–1 vs. 2–3] was significantly correlated with PFS (6.2 vs. 2.9 months, P=0.015) (Figure 2). No significant associations were found between PFS and liver metastasis (yes vs. no) (Figure 3) or brain metastasis (yes vs. no) (Figure 4).
Table 2
| Response | N | % |
|---|---|---|
| Clinical benefit response | 21 | 67.7 |
| Objective response | 8 | 25.8 |
| PR | 8 | 25.8 |
| SD | 13 | 41.9 |
| Progressive disease | 10 | 32.3 |
PR, partial response; SD, stable disease.

Safety
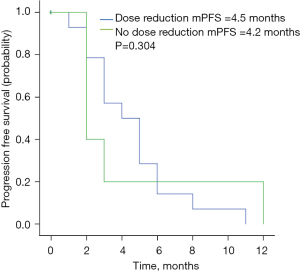
The AEs are shown in Table 3. The most common grade 3–4 AEs were diarrhea (19.4%), neutropenia (9.7%), and vomiting (6.5%). Dose reduction to 320 mg was conducted in 19.4% of all cases due to severe AEs mentioned above. No significant association was found between PFS and dose reduction (yes vs. no) (Figure 5).
Table 3
| AEs | Any grade (%) | Grade 3–4 (%) |
|---|---|---|
| Diarrhea | 81.6 | 19.4 |
| Vomiting | 61.3 | 6.5 |
| Fatigue | 48.4 | 0.0 |
| Neutropenia | 71.0 | 9.7 |
| Thrombocytopenia | 12.9 | 0.0 |
AEs, adverse events.
Discussion
The progress of anti-HER2 therapy has significantly improved the survival of patients with HER2-positive MBC. Patients progressing on anti-HER2 therapy should be offered additional anti-HER2 therapy with subsequent cytotoxic or endocrine treatment, since it is beneficial to continue blocking the HER2 pathway. The choice of the anti-HER2 agent will depend on the specific anti-HER2 therapy previously administered, country-specific availabilityand the time to progression (8). Pertuzumab, lapatinib, and T-DM1 are candidate regimens commonly recommended for failure of trastuzumab in HER2-positive MBC. T-DM1 could be considered for patients with HER2-positive MBC who have previously received trastuzumab and lapatinib for its favourable benefit than traditional combinations of chemotherapy (9,10). However, T-DM1 has already been approved for MBC in mainland China in 2021, but it’s not covered by medical insurance.
Neratinib is an irreversible ErbB receptor TKI. The mPFS of 8.8 months in the NALA trial and the mPFS of 12.9 months in the NEfERT-T trial suggests its promising anti-HER2 efficacy (11,12). Furthermore, the TBCRC022 trial showed that neratinib plus capecitabine had a mPFS of 3.1 months, an intracranial ORR of 33%, and an extracranial ORR of 43% in lapatinib-treated patients, demonstrating the efficacy of neratinib in lapatinib-treated patients (13).
Pyrotinib, serving as a TKI, directly blocks the intracellular domain and inhibits downstream pathway activation. Thus, it has the potential to overcome the drug resistance caused by traditional anti-HER2 drugs. Due to the limitations of drug selection, pyrotinib, with its novel anti-HER2 efficacy, has become a key approved regimen for treating HER2-positive MBC in trastuzumab-resistant patients in China. The results of this study demonstrated that pyrotinib-containing therapy achieved a mPFS of 4.5 months and an ORR of 25.8% in HER2-positive MBC. Compared to the mPFS of 11.1 months and the ORR of 68.5% achieved in the PHENIX study, the data in this study were less promising (6). The apparent difference was the patient characteristics between the 2 studies. The patients included in the PHENIX study had disease progression during or after treatment with trastuzumab plus no more than 2 lines of chemotherapy. The PHENIX study excluded patients previously treated with lapatinib. All patients in our study were heavily treated and failed in lapatinib-containing therapy. The patients in this study were recognized to be an anti-HER2 treatment refractory population.
One Chinese study reported the efficacy of pyrotinib-containing treatment in HER2-positive MBC lapatinib-naïve and lapatinib-treated patients. The mPFS was 5.4 months and the ORR was 23.2% for lapatinib-treated patients (14). The results in our study were comparable to the results of the lapatinib-treated subgroup. Thus, our results provide evidence in favor of the use of pyrotinib-containing therapy after failure of lapatinib-containing therapy. The number of patient samples in this study is too small, and a large sample study should be added in further study. Another limitation of this study was that few patients were treated with pertuzumab and T-DM1 before enrollment.
The incidence of brain metastasis is higher in the HER2-positive breast cancer subtype than other subtypes. Radiotherapy is a common treatment, and anti-HER2 small molecule TKIs have been used due to their ability to penetrate the blood brain barrier. The subgroup of 31 patients with brain metastasis receiving pyrotinib and capecitabine in the PHENIX trial had a mPFS of 6.9 months, and the patients with brain metastasis in the PHENIX study did not receive prior lapatinib-containing therapy. In the TBCRC022 trial, the subgroup of lapatinib-treated patients with brain metastasis receiving neratinib plus capecitabine had a mPFS of 3.1 months. In our study, there were only 6 brain metastasis patients, with a mPFS of 5.2 months. The results of our study suggest that pyrotinib-containing therapy may be an optimal treatment for HER2-positive MBC with brain metastasis after failure of lapatinib-containing therapy. The limitation of the small sample size of our study should be taken into consideration, and studies with a larger sample size are needed to confirm the results.
The pyrotinib-related AEs were generally tolerated, including diarrhea, neutropenia, vomiting, fatigue, and thrombocytopenia, among others. Diarrhea was the most common AE. The incidence of diarrhea observed in our study was similar to that of previous studies. It was controllable with loperamide treatment in 80% of patients. Neutropenia occurred in 71% of patients, which was higher than in the PHENIX study, probably because a larger proportion of patients in our study had received anti-HER2 therapy in combination with more than 2 lines of cytotoxic drugs before enrollment. Hand-foot syndrome in our study was less common than in the PHENIX study, as 74.2% of patients were not treated in combination with capecitabine. Pyrotinib in combination with cytotoxic drugs other than capecitabine also showed a good safety profile. And a long-term safety analysis should be reported after longer follow-up.
Conclusions
In conclusion, this study demonstrated the promising efficacy of pyrotinib-containing therapy for lapatinib-treated HER2-positive MBC in the third or later line settings. Pyrotinib-containing therapy was a safe treatment option with tolerable and controllable side effects. Prospective randomized controlled clinical studies with large sample sizes are needed to further investigate the role of pyrotinib in previously heavily-treated HER2-positive MBC patients.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (No. 81801725).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-21-3965/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-21-3965/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-21-3965/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Ruijin Hospital Shanghai Jiaotong University School of Medicine (Ethic Committee Reference Number 2021178). As this retrospective study did not harm the rights and health of patients, and protected their privacy and personal information, the ethics committee waived the requirement to obtain informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Onitilo AA, Engel JM, Greenlee RT, et al. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res 2009;7:4-13. [Crossref] [PubMed]
- Swain SM, Baselga J, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 2015;372:724-34. [Crossref] [PubMed]
- Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 2012;367:1783-91. [Crossref] [PubMed]
- Ma F, Li Q, Chen S, et al. Phase I Study and Biomarker Analysis of Pyrotinib, a Novel Irreversible Pan-ErbB Receptor Tyrosine Kinase Inhibitor, in Patients With Human Epidermal Growth Factor Receptor 2-Positive Metastatic Breast Cancer. J Clin Oncol 2017;35:3105-12. [Crossref] [PubMed]
- Ma F, Ouyang Q, Li W, et al. Pyrotinib or Lapatinib Combined With Capecitabine in HER2-Positive Metastatic Breast Cancer With Prior Taxanes, Anthracyclines, and/or Trastuzumab: A Randomized, Phase II Study. J Clin Oncol 2019;37:2610-9. [Crossref] [PubMed]
- Yan M, Bian L, Hu X, et al. Pyrotinib plus capecitabine for human epidermal growth factor receptor 2-positive metastatic breast cancer after trastuzumab and taxanes (PHENIX): a randomized, double-blind, placebo-controlled phase 3 study. Transl Breast Cancer Res 2020;1:13. [Crossref]
- Xu B, Yan M, Ma F, et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol 2021;22:351-60. [Crossref] [PubMed]
- Untch M, Würstlein R, Marschner N, et al. 4th International Consensus Conference on Advanced Breast Cancer (ABC4), Lisbon, November 4, 2017: ABC4 Consensus: Assessment by a Panel of German Experts. Geburtshilfe Frauenheilkd 2018;78:469-80. [Crossref] [PubMed]
- Krop IE, Kim SB, González-Martín A, et al. Trastuzumab emtansine versus treatment of physician's choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol 2014;15:689-99. [Crossref] [PubMed]
- Krop IE, Kim SB, Martin AG, et al. Trastuzumab emtansine versus treatment of physician's choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol 2017;18:743-54. [Crossref] [PubMed]
- Saura C, Oliveira M, Feng YH, et al. Neratinib Plus Capecitabine Versus Lapatinib Plus Capecitabine in HER2-Positive Metastatic Breast Cancer Previously Treated With ≥2 HER2-Directed Regimens: Phase III NALA Trial. J Clin Oncol 2020;38:3138-49. [Crossref] [PubMed]
- Awada A, Colomer R, Inoue K, et al. Neratinib Plus Paclitaxel vs Trastuzumab Plus Paclitaxel in Previously Untreated Metastatic ERBB2-Positive Breast Cancer: The NEfERT-T Randomized Clinical Trial. JAMA Oncol 2016;2:1557-64. [Crossref] [PubMed]
- Freedman RA, Gelman RS, Anders CK, et al. TBCRC 022: A Phase II Trial of Neratinib and Capecitabine for Patients With Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer and Brain Metastases. J Clin Oncol 2019;37:1081-9. [Crossref] [PubMed]
- Lin Y, Lin M, Zhang J, et al. Real-World Data of Pyrotinib-Based Therapy in Metastatic HER2-Positive Breast Cancer: Promising Efficacy in Lapatinib-Treated Patients and in Brain Metastasis. Cancer Res Treat 2020;52:1059-66. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)


