Opioid withdrawal presenting only nausea during tapering of oxycodone after celiac plexus block: a case report
Introduction
Pancreatic cancer is recognized as one of the most painful types of malignancy. Most patients with severe cancer pain are treated with maximum opioid for pain relief. However, they may suffer from side effects of opioid use. Celiac plexus block (CPB) is an effective treatment for patients with visceral pain arising from malignancy, such as pancreatic cancer (1,2). CPB may allow for a reduction in opioid dosage, and may alleviate some of the unwanted side effects of opioid use (1,2).
There is a substantial risk of physical dependence and subsequent withdrawal symptoms after reduction and/or discontinuation of opioid. The symptoms of opioid withdrawal are various, including agitation, anxiety, muscle aches, sweating, yawning, diarrhea, dilated pupils, goose bumps, nausea, and vomiting (3-5).
Here, we report a case of advanced pancreatic cancer with persistent nausea before and after tapering of oxycodone following CPB. The patient presented only nausea, leading to a delay in diagnosis of opioid withdrawal. The symptom in the present case was nonspecific for opioid withdrawal and we reviewed the symptoms.
Case presentation
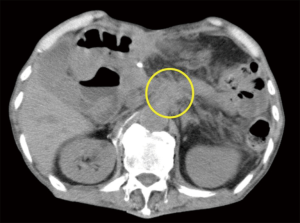
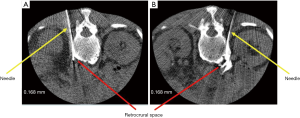
A 70-year-old man diagnosed with advanced pancreatic cancer was referred to our hospital because of severe back pain. He had been administered oxycodone at 240 mg per day for 2 weeks. However, his pain evaluated on a numerical rating scale (NRS, 0: no pain; 10: worst pain imaginable) was 8−10, and he presented severe drowsiness, nausea, and fatigue due to the high dosage of oxycodone. As radiographic findings revealed tumor infiltrating the celiac plexus (Figure 1), computed tomography (CT)-guided CPB by the posterior approach was planned. The patient was placed in a prone position on a CT table. CPB was performed using the paravertebral approach at the level of L1 vertebra. A needle tip of left-sided block was positioned in the left retrocrural space (Figure 2A), and right-sided was also in the right retrocrural space (Figure 2B). We injected 10 mL of 99.9% ethanol into the each retrocrural space.
His pain disappeared on the day of CPB treatment. We planned to taper the opioid as soon as possible, and reduced the oxycodone from 240 to 160 mg/day. The next day, he reported no pain but complained of more severe nausea compared to that before CPB. Two days later, oxycodone was further reduced to 80 mg/day. However, he still complained of nausea, which resulted in complete loss of appetite. Physical examination showed no abnormalities and blood chemistry examination revealed no abnormalities in electrolytes or hepatic and renal functions. We administered metoclopramide, diphenhydramine salicylate, and olanzapine, but these treatments failed to improve the nausea. In addition, brain CT showed no abnormalities. Five days later, he appeared to be irritable in addition to nausea, which suggested symptoms of opioid withdrawal. Administration of fast-release oxycodone (Oxinome®) at a dose of 10 mg immediately improved his nausea and irritability. These findings convinced us that his symptoms were due to opioid withdrawal. We gradually reduced the dose of oxycodone over 2 weeks. Finally, the dose of oxycodone reached 10 mg without the development of pain. Time course of nausea and back pain before and after CPB described above was summarized in Figure 3. The complained several times of nausea, but fast-release oxycodone was effective for the relief. He had not presented with typical symptoms of opioid withdrawal, such as anxiety, insomnia, rhinorrhea, muscle aches, heart rate fluctuations, sweating, or abnormalities in blood pressure or body temperature. The patient died due to progressive disease of pancreatic cancer 2.5 months after CPB but showed neither complaints of back pain or requirement for increased opioids.

Discussion
The present case developed opioid withdrawal during tapering of oxycodone after CPB. In general, withdrawal symptoms, especially in the first 24 hours, include yawning, lacrimation, rhinorrhea, sweating, tremors, cutis anserina, mydriasis, lack of composure, and insomnia (3-5). Subsequently, elevated body temperature, increased respiratory rate, and elevation of blood pressure are observed. Nausea and vomiting are considered to be symptoms occurring during the late phase of opioid withdrawal (3-5). The present case exhibited only nausea from the onset of opioid withdrawal without any other suspicious symptoms, which is an extremely rare clinical manifestation. As another symptom, irritability was observed on day 5 after nerve block, which was the only suspicious symptom for opioid withdrawal during the clinical course.
In general, the cause of nausea in palliative care patients with advanced cancer should be considered gastritis, constipation, gastrointestinal tract obstruction, electrolyte abnormalities, infection, liver or renal dysfunction, brain hypertension, meningitis, mental illness and so on in addition to drug-induced, e.g., opioid (6). However no abnormalities were found, including physical examinations, blood chemistry and brain CT etc. in the present case. Currently widely available antiemetics such as metoclopramide, dexamethasone, haloperidol, diphenhydramine salicylate and olanzapine were administered, but failed to improve his nausea. In addition, we considered the possibility that nausea after CPB was a persistent side effect of the still high dose of oxycodone even after the reduced dose. However, although the patient did not complain of nausea until a dose of 160 mg of oxycodone, the nausea was not alleviated and worsened after dosage reduction to 80 mg.
Fast-release oxycodone was useful for improvement of nausea while receiving 80 mg of oxycodone. Therefore, the nausea in the present case was thought to be related to opioid withdrawal. Thus, physicians should be aware that, although nausea is a common side effect of opioid, the present case suggested that nausea could be a symptom of opioid withdrawal.
There are no established methods for reducing opioid dosage after CPB, because the reduction is dependent on the effect of CPB. However, it has been suggested that the dosage should be decreased by 50% every 3 days to 1 week after CPB (7). Based on this suggestion, oxycodone dose was decreased from 240 mg/day by 80 mg/day every 3 days, which was consistent with the pattern in the present case. In addition to dose reduction, it is well known that withdrawal symptoms usually occur after long-term administration of opioids, but the type and intensity of symptoms differ (3-5). In contrast, these symptoms may occur even after short-term administration or small dosages (3). Thus, the present case indicated that clinical manifestations of opioid withdrawal are complex and we should take into consideration the presence of opioid withdrawal when reducing opioid dosage.
In conclusion, opioid withdrawal refers to a wide range of symptoms. To our knowledge, there have been no previous reports describing nausea as the sole symptom of opioid withdrawal. If opioid side effects, such as nausea and vomiting, do not improve after dosage reduction, as in the present case, the possibility of withdrawal syndrome should also be considered.
Acknowledgements
The authors thank Masahiro Kurozumi, M.D. (Department of Radiology, Shinshu University School of Medicine, Matsumoto, Japan), for his help in the CPB.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Zhong W, Yu Z, Zeng JX, et al. Celiac plexus block for treatment of pain associated with pancreatic cancer: a meta-analysis. Pain Pract 2014;14:43-51. [PubMed]
- Arcidiacono PG, Calori G, Carrara S, et al. Celiac plexus block for pancreatic cancer pain in adults. Cochrane Database Syst Rev 2011.CD007519. [PubMed]
- Mattick RP, Hall W. Are detoxification programmes effective? Lancet 1996;347:97-100. [PubMed]
- Plasencia AM, Furbee RB. Opioids. In: Wolfson AB, Hendey GW, Ling LJ, et al., editors. Harwood-Nuss’ Clinical Practice of Emergency Medicine. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2009:chap 289.
- Doyon S. Opiods. In: Tintinalli JE, Kelen GD, Stapczynski JS, et al. editors. Emergency Medicine: A Comprehensive Study Guide. 6th ed. New York, NY: McGraw-Hill, 2004:chap 167.
- Glare P, Miller J, Nikolova T, et al. Treating nausea and vomiting in palliative care: a review. Clin Interv Aging 2011;6:243-59. [PubMed]
- Hegmann KT, Weiss MS, Bowden K, et al. ACOEM practice guidelines: opioids for treatment of acute, subacute, chronic, and postoperative pain. J Occup Environ Med 2014;56:e143-59. [PubMed]


