Enteral and parenteral nutrition in cancer patients: a systematic review and meta-analysis
Background
Energy imbalance, typically caused by a decrease in food intake, is responsible for weight loss as body tissues are consumed for fuel (1). In cancer patients, weight loss is an ominous sign predicting disease progression and shortened survival time (2,3). As a result, providing nutrition support for cancer patients has been proposed as a logical approach for improving clinical outcomes (1). However, some studies have reported increased complications and costs (4,5). However the readers are cautioned that the world of clinical nutrition is markedly changed in the last 3 decades, i.e., many technological innovations have significantly increased the cost effectiveness of nutrition support (commercial ‘all-in-one bags’, new enteral and parenteral formulas, peripheral insertion, new materials for venous and enteral accesses, etc.), whereas new strategies have successfully minimized the risk of complications (standardized “bundles” of evidence-based interventions, strict policies of antisepsis, education of healthcare operators, etc.).
Nutrition support can be given to patients through enteral nutrition (EN) or parenteral nutrition (PN) (6). EN may be the preferred method of nutrition support, not only because of lower costs and fewer complications, but also due to the perceived better outcomes (6). Previously, meta-analyses by Heyland et al. and Braunschweig et al. conducted in 1998 and 2001, respectively, have evaluated the outcomes of EN, in both standard care (SC) and tube feeding (TF), compared to PN (7,8). Braunschweig et al. reported a trend for a lower risk of infection in the EN study population, while Heyland et al. claimed lower rates of complications in the PN study population (7,8). Both studies also disagreed on the mortality rates in response to the different treatment options (7,8).
The potential adverse consequences of PN and EN make it important to establish the therapeutic benefits of both nutrition support options before recommending their routine use in cancer patients (1). To date, a meta-analysis focusing primarily on the outcomes of EN and PN in the cancer setting has not been conducted. The purpose of the following review was to compare the outcomes of PN and EN in cancer patients.
Materials and methods
Search strategy
A literature search was conducted in Ovid MEDLINE and OLDMEDLINE from 1946 to July Week 2 2015, Embase Classic and Embase from 1947 to 2015 Week 29, and Cochrane Central Register of Controlled Trials up until June 2015. Search terms included “PN”, “comparative study”, and “EN”. The search was limited to English-language studies and randomized controlled trials (RCTs). The complete search strategy is displayed in Figure 1. Reference lists from studies identified by the search were examined as well. Titles and abstracts were screened to identify references that were relevant for full-text review, based on pre-specified selection criteria for full-text review. Articles were identified for full-text review if the title or abstract included mentioning of parenteral and EN as two separate nutrition support treatment arms. Duplicates of articles found in each database were excluded.
Selection criteria for meta-analysis
Studies were included if over 50% of the study population had some type of cancer. Non-original research and small-sized trials (<5 patients) were excluded. Studies that did specify the medical procedure but not the medical diagnoses of the patient population were also excluded.
Endpoints
The primary endpoints were the percentage of patients that experienced no infection, nutrition support complications, major complications and mortality. The outcomes of thirteen studies (9-21) as reported by Braunschweig et al. (8) were recorded, except for the “Other Complications” heading in their table.
“Minor infections” as reported by studies were recorded under infection. For studies that reported the breakdown of infection complications, we simply recorded the number of patients that experienced wound infection, pneumonia and sepsis. Nutrition support complications were recorded as reported in the study or the summation of nausea and vomiting events were recorded. Major complications or morbidity, as reported in studies, were noted as major complications. Mortality rates were noted as mentioned in the literature.
The type of EN, TF or SC, was also noted. Additionally, we noted if there were members of the study population that were malnourished, or deemed protein-energy malnutrition (PEM), via binary options of yes or no. For studies that did not mention PEM, we assumed there were no patients malnourished as we postulated that such demographics would certainly be reported if they existed.
Subgroup analysis
We stratified primary endpoints according to type of EN for subgroup analysis, grouping studies into either TF or SC as defined in the publications. Additionally, subgroup analysis was conducted on whether studies were composed of PEM patients or not.
Statistical analysis
Statistical analyses were conducted using Review Manager (RevMan 5.3) for Cochrane IMS. The Mantel-Haenszel method was applied and a random effect analysis model was used to generate risk ratios (RR), and their accompanying 95% confidence intervals (CIs). A P value of less than 0.05 was considered statistically significant in the test for overall effect and a heterogeneity test with p-value greater than 0.05 was considered suitable. For all endpoints in the forest plots, we used the number of patients that did not experience the outcomes as the event numbers. This allowed for all endpoints to be greater than 0, thus allowing for calculable RR for all studies.
Results
The literature search yielded 674 articles, with 186 from MEDLINE, 277 from EMBASE, and 211 from Cochrane. An additional 68 were identified from the references of the papers. Of the 661 titles and abstracts screened (9-33), 36 were included for the meta-analysis (Figure 2) (34-44).
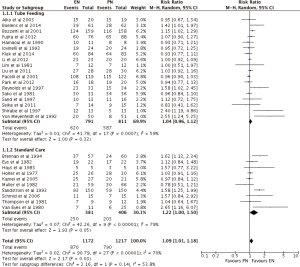
Infection
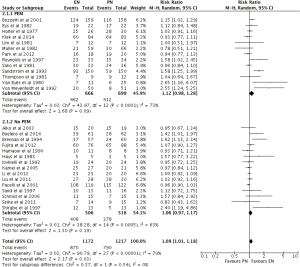
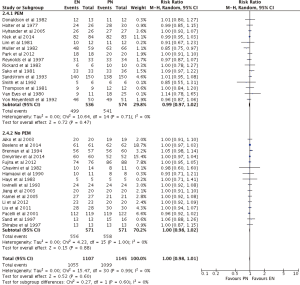
EN was statistically superior to PN, with a point estimate of RR as 1.09, and 95% CI from 1.01 to 1.18 (P=0.03) (Figures 3,4). However, neither EN nor PN were superior in subgroup analysis of TF (RR =1.04; 95% CI: 0.96–1.12; P=0.32) and SC (RR =1.22; 95% CI: 1.00–1.50; P=0.05) (Figure 3). Subgroup analysis of studies with (RR =1.12; 95% CI: 0.98–1.12; P=0.09) and without (RR =1.06; 95% CI: 0.97–1.17; P=0.19) PEM patients showed no difference, with respect to infection, between EN and PN (Figure 4).
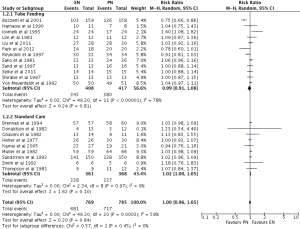
Nutrition support complications
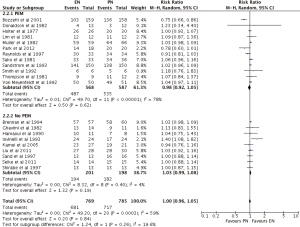
Overall, EN and PN achieved the same nutrition support complications (RR =1.00; 95% CI: 0.96–1.05; P=0.83) (Figures 5,6). Subgroup analysis of EN types showed TF (RR =0.99; 95% CI: 0.91–1.08; P=0.81) and SC (RR =1.02; 95% CI: 1.00–1.05; P=0.10) to produce similar outcomes to PN (Figure 5). Subgroup analysis of PEM patients revealed indifference as well between EN and PN, with PEM patients (RR =0.98, 95% CI: 0.92–1.05, P=0.62) and no PEM patients (RR =1.03, 95% CI: 0.99–1.08, P=0.19) achieving similar outcomes (Figure 6).
Major complications
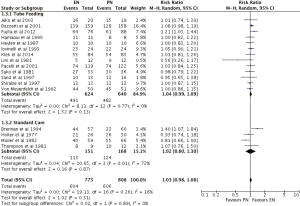
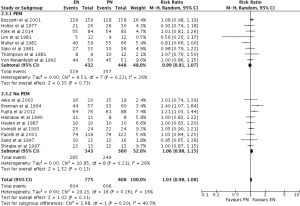
There was no difference between EN and PN, with respect to major complications (RR =1.03; 95% CI: 0.98–1.08; P=0.31) (Figures 7,8). Analysis by types of EN also showed no superiority of either treatment, TF (RR =1.04; 95% CI: 0.99–1.09; P=0.13) and SC (RR =1.02; 95% CI: 0.80–1.30; P=87), in comparison to PN (Figure 7). Furthermore, subgroup analysis of studies containing no PEM (RR =1.06; 95% CI: 0.98–1.15; P=0.13) and PEM (RR =0.99; 95% CI: 0.91–1.07; P=0.73) patients showed indifference (Figure 8).
Mortality
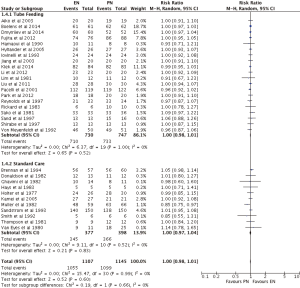
In terms of survival, neither EN nor PN were found to be superior (RR =1.00; 95% CI: 0.97–1.04; P=0.60) (Figures 9,10). Subgroup analysis of TF (RR =1.00; 95% CI: 0.98–1.01; P=0.52) and SC (RR =1.00; 95% CI: 0.97–1.04; P=0.83) (Figure 9) showed no survival differences between EN and PN, as did subgroup analysis of studies that contained (RR =0.99; 95% CI: 0.97–1.02; P=0.47) and did not contain (RR =1.00; 95% CI: 0.98–1.02; P=0.88) PEM patients (Figure 10).
Heterogeneity
Two of four primary analyses between EN and PN had unsuitable levels of heterogeneity (Infection: P<0.00001; Nutrition support complications: P=0.0003) (Figures 3-6). Seven of sixteen subgroup analyses of EN and PN also had unsuitable levels of heterogeneity, namely infections of the TF (P=0.0007), SC (P<0.00001), PEM (P<0.0001), and no PEM (P=0.0005) cohorts, nutrition support complications of the TF (P<0.00001) and PEM (P<0.00001) cohorts, and major complications with respect to SC (P=0.01) (Figures 3-7). The remaining two primary analyses and eleven subgroup analyses had satisfactory levels of heterogeneity (P values from 0.21 to 1.00) (Figures 5-10).
Discussion
During the last 15 years, conflicting meta-analysis results regarding the benefits of EN vs. PN in different ICU, surgical or cancer populations were published. A common reason a cancer patient may need nutrition support is due to negative side effects of the anticancer treatments (surgery, chemotherapy, and radiation therapy). In such patients, the European guidelines recommend ‘EN if oral nutrition remains inadequate despite nutritional interventions, and PN if EN is not sufficient or feasible’ (45). Moreover, independently regardless of whether receiving or not receiving anticancer therapies, the administration of PN depends on the oncological diagnosis. The most frequent diagnoses among patients with PN were tumors of the gastrointestinal tract, i.e., gastric carcinoma, colorectal carcinoma, and pancreatic carcinoma. Besides, the work of Orrevall et al. (46) showed that nausea, vomiting, and obstructions were the most common indications for PN in palliative patients. As in many other papers of this type, any conclusion is hardly generalizable to the overall cancer patient population. EN and PN are competitors in the choice of way to deliver nutrition support in cancer patients but have specific indications and contraindications.
This is the first study to our knowledge to review and compare the outcomes of EN and PN in cancer patients. A meta-analysis conducted by Braunschweig et al. in 2001 reported in a subgroup analysis that EN was superior to PN in the cancer population with respect to less infection and other complications in the TF population (8), which was not shown in our meta-analysis. However, it should be noted that while Braunschweig et al.’s study only included eight studies in their subgroup analyses of cancer patients (8), our meta-analysis comprises of a total of 36 studies. The result of our study does confirm Braunschweig et al.’s finding that the infection of EN patients in general, regardless of type, are less likely to contract infections. The most feared and relevant complications of PN are catheter-related bloodstream infections. Indeed, nowadays all cancer patients have a CVC, independently regardless of whether receiving or not receiving PN.
Several studies have reported that PN patients receive more calories than EN patients (10,23,27,44). As PN has been shown to provide more calories for patients, it has been hypothesized that PN is more effective for malnourished patients when compared to EN. Accordingly, some institutions have made it common practice to assign malnourished patients to PN (26,27,44).
Although PN allows for easy administration of a predetermined amount of calories, micronutrients and substrates, it has been reported to also encourages gut atrophy and bacterial translocation due to the absence of enteral food elements (7,47-49), in addition to potentially stimulating tumor growth (50-54). In contrast, EN, specifically TF, is cheaper and has fewer complications, but has been reported to also be associated with higher mortality rates, specifically in the malnourished population (8). Our study finds that in the cancer population, EN does indeed result in fewer infection, but does not have higher mortality rates or major complications associated with it. Since 2009, the European guidelines recommend that ‘Although PN supplies nutrients to the tumor, there is no evidence that this has deleterious effects on the outcome. This consideration should therefore have no influence on the decision to feed a cancer patient when PN is clinically indicated’ (55).
While the existing literature reveals that the additional calories provided by PN may not actually translate to better survival rates (56,57), there is no consensus on the practice. For instance, a study by Bozzetti et al. conducted a RCT of malnourished cancer patients comparing PN and EN treatments and found that 9% of EN cases required switchover to PN (24), as deemed necessary by physicians. Clinicians may still have a general perception that higher caloric intake will improve survival. In contrast, the study by Bozzetti et al. speculated that patients may actually have better survival rates with EN (24). Our meta-analysis shows no significant advantage in survival for patients receiving PN.
In comparison to EN, PN has also been reported to require less time in improving a patient’s nutritional state and to be more beneficial in the cancer surgery setting (14,24). The shorter timeframe during preoperative and postoperative stay is beneficial for hospitals in aiming to keep hospital stay to a minimum due to limited hospital beds (24). However, PN has been reported to be over twice the cost of EN (10); thus, despite shorter hospital stays, patients receiving PN may incur greater financial costs (34). Evidence showed that nutrition support is a relatively cheap adjuvant therapy if compared to other anticancer therapies but a prolonged in-hospital length of stay may be more expensive than PN administration.
Of note, patients receiving EN may experience a decreased flexibility when compared to PN patients since oral feeding must be withheld for some preoperative diagnostic procedures (14). The ability to continue artificial nutrition uninterrupted via PN at all treatment stages may partially justify its higher overall price. Still, this meta-analysis shows that, with respect to complications (both in nutrition support and major complications) and mortality, there is no added benefit in receiving PN instead of EN.
This review was not without limitations. The text of two studies (17,19) was not found, and hence verification of the data supplied by Braunschweig et al. (8) was not possible. Additionally, one study (25) was in the form of an abstract. Furthermore, the reporting across studies was not standardized: there were different definitions and recording methods for the infections, nutrition support complications and major complications outcomes. Moreover, while some studies reported the number of episodes reported per outcome (32), other studies solely reported the number of patients who experienced the outcome (22,23). Additionally, some studies defined “Major Complications” differently, resulting in difficult cross-comparison among all studies included.
Conclusions
In conclusion, this systematic review highlights that neither PN nor EN are superior with respect to nutrition support complications, major complications and mortality. EN, the conglomerate of TF and SC, was favoured over PN with respect to less infection. The perceived advantages of PN in lower mortality rates and fewer complications due to higher and more efficient caloric intake are not confirmed in the cancer population.
Acknowledgements
We thank the generous support of Bratty Family Fund, Michael and Karyn Goldstein Cancer Research Fund, Joey and Mary Furfari Cancer Research Fund, Pulenzas Cancer Research Fund, Joseph and Silvana Melara Cancer Research Fund, and Ofelia Cancer Research Fund.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Klein S, Koretz RL. Nutrition support in patients with cancer: what do the data really show? Nutr Clin Pract 1994;9:91-100. [PubMed]
- Dewys WD, Begg C, Lavin PT, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med 1980;69:491-7. [PubMed]
- Stanley KE. Prognostic factors for survival in patients with inoperable lung cancer. J Natl Cancer Inst 1980;65:25-32. [PubMed]
- Mullen JL. Complications of total parenteral nutrition in the cancer patient. Cancer Treat Rep 1981;65 Suppl 5:107-13. [PubMed]
- Twomey PL, Patching SC. Cost-effectiveness of nutritional support. JPEN J Parenter Enteral Nutr 1985;9:3-10. [PubMed]
- Altintas ND, Aydin K, Türkoğlu MA, et al. Effect of enteral versus parenteral nutrition on outcome of medical patients requiring mechanical ventilation. Nutr Clin Pract 2011;26:322-9. [PubMed]
- Heyland DK, MacDonald S, Keefe L, et al. Total parenteral nutrition in the critically ill patient: a meta-analysis. JAMA 1998;280:2013-9. [PubMed]
- Braunschweig CL, Levy P, Sheean PM, et al. Enteral compared with parenteral nutrition: a meta-analysis. Am J Clin Nutr 2001;74:534-42. [PubMed]
- Brennan MF, Pisters PW, Posner M, et al. A prospective randomized trial of total parenteral nutrition after major pancreatic resection for malignancy. Ann Surg 1994;220:436-41; discussion 441-4. [PubMed]
- Hamaoui E, Lefkowitz R, Olender L, et al. Enteral nutrition in the early postoperative period: a new semi-elemental formula versus total parenteral nutrition. JPEN J Parenter Enteral Nutr 1990;14:501-7. [PubMed]
- Holter AR, Fischer JE. The effects of perioperative hyperalimentation on complications in patients with carcinoma and weight loss. J Surg Res 1977;23:31-4. [PubMed]
- Iovinelli G, Marsili I, Varrassi G. Nutrition support after total laryngectomy. JPEN J Parenter Enteral Nutr 1993;17:445-8. [PubMed]
- Lim ST, Choa RG, Lam KH, et al. Total parenteral nutrition versus gastrostomy in the preoperative preparation of patients with carcinoma of the oesophagus. Br J Surg 1981;68:69-72. [PubMed]
- Müller JM, Brenner U, Dienst C, et al. Preoperative parenteral feeding in patients with gastrointestinal carcinoma. Lancet 1982;1:68-71. [PubMed]
- Reynolds JV, Kanwar S, Welsh FK, et al. 1997 Harry M. Vars Research Award. Does the route of feeding modify gut barrier function and clinical outcome in patients after major upper gastrointestinal surgery? JPEN J Parenter Enteral Nutr 1997;21:196-201. [PubMed]
- Sako K, Loré JM, Kaufman S, et al. Parenteral hyperalimentation in surgical patients with head and neck cancer: a randomized study. J Surg Oncol 1981;16:391-402. [PubMed]
- Sand J, Luostarinen M, Matikainen M. Enteral or parenteral feeding after total gastrectomy: prospective randomised pilot study. Eur J Surg 1997;163:761-6. [PubMed]
- Sandström R, Drott C, Hyltander A, et al. The effect of postoperative intravenous feeding (TPN) on outcome following major surgery evaluated in a randomized study. Ann Surg 1993;217:185-95. [PubMed]
- Shirabe K, Matsumata T, Shimada M, et al. A comparison of parenteral hyperalimentation and early enteral feeding regarding systemic immunity after major hepatic resection--the results of a randomized prospective study. Hepatogastroenterology 1997;44:205-9. [PubMed]
- Thompson BR, Julian TB, Stremple JF. Perioperative total parenteral nutrition in patients with gastrointestinal cancer. J Surg Res 1981;30:497-500. [PubMed]
- Von Meyenfeldt MF, Meijerink WJ, Rouflart MM, et al. Perioperative nutritional support: a randomised clinical trial. Clin Nutr 1992;11:180-6. [PubMed]
- Aiko S, Yoshizumi Y, Matsuyama T, et al. Influences of thoracic duct blockage on early enteral nutrition for patients who underwent esophageal cancer surgery. Jpn J Thorac Cardiovasc Surg 2003;51:263-71. [PubMed]
- Boelens PG, Heesakkers FF, Luyer MD, et al. Reduction of postoperative ileus by early enteral nutrition in patients undergoing major rectal surgery: prospective, randomized, controlled trial. Ann Surg 2014;259:649-55. [PubMed]
- Bozzetti F, Braga M, Gianotti L, et al. Postoperative enteral versus parenteral nutrition in malnourished patients with gastrointestinal cancer: a randomised multicentre trial. Lancet 2001;358:1487-92. [PubMed]
- Dmytriiev D, Katilov O, Dmytriiev K, et al. The role of perioperative enteral and parenteral nutrition treatment in children with abdominal cancer. Clin Nutr 2014;33:S134-5.
- Donaldson SS, Wesley MN, Ghavimi F, et al. A prospective randomized clinical trial of total parenteral nutrition in children with cancer. Med Pediatr Oncol 1982;10:129-39. [PubMed]
- van Eys J, Wesley MN, Cangir A, et al. Safety of intravenous hyperalimentation in children with malignancies: a cooperative group trial. JPEN J Parenter Enteral Nutr 1982;6:291-4. [PubMed]
- Fujita T, Daiko H, Nishimura M. Early enteral nutrition reduces the rate of life-threatening complications after thoracic esophagectomy in patients with esophageal cancer. Eur Surg Res 2012;48:79-84. [PubMed]
- Ghavimi F, Shils ME, Scott BF, et al. Comparison of morbidity in children requiring abdominal radiation and chemotherapy, with and without total parenteral nutrition. J Pediatr 1982;101:530-7. [PubMed]
- Hays DM, Merritt RJ, White L, et al. Effect of total parenteral nutrition on marrow recovery during induction therapy for acute nonlymphocytic leukemia in childhood. Med Pediatr Oncol 1983;11:134-40. [PubMed]
- Heylen AM, Lybeer MB, Penninckx FM, et al. Parenteral versus needle jejunostomy nutrition after total gastrectomy. Clin Nutr 1987;6:131-6.
- Hyltander A, Bosaeus I, Svedlund J, et al. Supportive nutrition on recovery of metabolism, nutritional state, health-related quality of life, and exercise capacity after major surgery: a randomized study. Clin Gastroenterol Hepatol 2005;3:466-74. [PubMed]
- Jiang XH, Li N, Li JS. Intestinal permeability in patients after surgical trauma and effect of enteral nutrition versus parenteral nutrition. World J Gastroenterol 2003;9:1878-80. [PubMed]
- Kamei H, Hachisuka T, Nakao M, et al. Quick recovery of serum diamine oxidase activity in patients undergoing total gastrectomy by oral enteral nutrition. Am J Surg 2005;189:38-43. [PubMed]
- Klek S, Szybinski P, Szczepanek K. Perioperative immunonutrition in surgical cancer patients: a summary of a decade of research. World J Surg 2014;38:803-12. [PubMed]
- Li G, Gu R, Wen X, et al. The effect of early enteral nutrition on hyperthermic intraoperative intraperitoneal chemotherapy-induced mucosal permeability following gastrectomy. JPEN J Parenter Enteral Nutr 2012;36:213-8. [PubMed]
- Liu C, Du Z, Lou C, et al. Enteral nutrition is superior to total parenteral nutrition for pancreatic cancer patients who underwent pancreaticoduodenectomy. Asia Pac J Clin Nutr 2011;20:154-60. [PubMed]
- Pacelli F, Bossola M, Papa V, et al. Enteral vs parenteral nutrition after major abdominal surgery: an even match. Arch Surg 2001;136:933-6. [PubMed]
- Park JS, Chung HK, Hwang HK, et al. Postoperative nutritional effects of early enteral feeding compared with total parental nutrition in pancreaticoduodectomy patients: a prosepective, randomized study. J Korean Med Sci 2012;27:261-7. [PubMed]
- Rickard KA, Detamore CM, Coates TD, et al. Effect of nutrition staging on treatment delays and outcome in Stage IV neuroblastoma. Cancer 1983;52:587-98. [PubMed]
- Schmid I, Schmitt M, Streiter M, et al. Parenteral nutrition is not superior to replacement fluid therapy for the supportive treatment of chemotherapy induced oral mucositis in children. Eur J Cancer 2006;42:205-11. [PubMed]
- Seike J, Tangoku A, Yuasa Y, et al. The effect of nutritional support on the immune function in the acute postoperative period after esophageal cancer surgery: total parenteral nutrition versus enteral nutrition. J Med Invest 2011;58:75-80. [PubMed]
- Smith DE, Handy DJ, Holden CE, et al. An investigation of supplementary nasogastric feeding in malnourished children undergoing treatment for malignancy: results of a pilot study. J Hum Nutr Diet 1992;5:85-91.
- van Eys J, Copeland EM, Cangir A, et al. A clinical trial of hyperalimentation in children with metastatic malignancies. Med Pediatr Oncol 1980;8:63-73. [PubMed]
- Arends J. ESPEN Guidelines: nutrition support in cancer. Available online: http://www.espen.org/presfile/Arends_J_2014.pdf
- Orrevall Y, Tishelman C, Permert J, et al. A national observational study of the prevalence and use of enteral tube feeding, parenteral nutrition and intravenous glucose in cancer patients enrolled in specialized palliative care. Nutrients 2013;5:267-82. [PubMed]
- Iapichino G, Gattinoni L, Solca M, et al. Protein sparing and protein replacement in acutely injured patients during TPN with and without amino acid supply. Intensive Care Med 1982;8:25-31. [PubMed]
- ASPEN Board of Directors and the Clinical Guidelines Task Force. Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. JPEN J Parenter Enteral Nutr 2002;26:1SA-138SA.
- Jeejeebhoy KN. Total parenteral nutrition: potion or poison? Am J Clin Nutr 2001;74:160-3. [PubMed]
- Steiger E, Oram-Smith J, Miller E, et al. Effects of nutrition on tumor growth and tolerance to chemotherapy. J Surg Res 1975;18:455-66. [PubMed]
- Popp MB, Wagner SC, Brito OJ. Host and tumor responses to increasing levels of intravenous nutritional support. Surgery 1983;94:300-8. [PubMed]
- Popp MB, Kirkemo AK, Morrison SD, et al. Tumor and host carcass changes during total parenteral nutrition in an anorectic rat-tumor system. Ann Surg 1984;199:205-10. [PubMed]
- Ota DM, Copeland EM 3rd, Strobel HW Jr, et al. The effect of protein nutrition on host and tumor metabolism. J Surg Res 1977;22:181-8. [PubMed]
- Heys SD, Park KG, McNurlan MA, et al. Stimulation of protein synthesis in human tumours by parenteral nutrition: evidence for modulation of tumour growth. Br J Surg 1991;78:483-7. [PubMed]
- Bozzetti F, Arends J, Lundholm K, et al. ESPEN Guidelines on Parenteral Nutrition: non-surgical oncology. Clin Nutr 2009;28:445-54. [PubMed]
- Nixon DW, Moffitt S, Ansley J, et al. Parenteral nutrition supplementation in cancer. International symposium on clinical nutrition. London: Royal College of Physicians, 1980.
- Bothe A, Valerio D, Bistrian BR, et al. Randomized control trial of hospital nutritional support during abdominal radiotherapy. J Parenter Enteral Nutr 1979;3:292.



