
Imaging features inferring symptom onset due to spinal metastasis progression: a preliminary study
Introduction
Recent advances in cancer treatments offer improved overall survival for cancer patients. Some are associated with disease-free survival after such treatment, without any signs or symptoms of cancer, but others who have received long-term anti-cancer treatments are not disease-free. The number of patients with spinal metastases are increasing as the survival rate of patients with cancer increases. Spinal metastases usually result in spinal fragility and instability. Spinal instability involves the loss of the normal range of motion and the risk of fracture or collapse under mechanical stress. Subsequently, spinal metastasis could cause intractable pain and neurological disorders, which not only lead to deterioration of the activities of daily living (ADLs) but also profoundly impair health-related quality of life (QOL).
Spinal metastasis-related pain includes neuropathic pain caused by lesions on the spinal cord and/or nerve roots, and local nociceptive pain due to direct bone injury. Further, spinal metastasis-related neuropathic pain consists of pain at (at-level) as well as below (below-level) the injured level (1,2). While at-level pain arises from lesioning of nerve roots and/or the spinal cord and is perceived at the corresponding segment, below-level pain is a central pain directly caused by damage to the spinal cord, and is often accompanied by complete or incomplete lower limb paraplegia. Anecdotal evidence suggests that central neuropathic pain begins as early as 1 month after spinal cord injury. Acute neuropathic pain often does not resolve by itself. Treatments in this early window might prevent progression to chronic central neuropathic pain. However, in contrast to local nociceptive pain, neuropathic pain is, in general, resistant to pharmacotherapies, including opioids; therefore, it should be prevented if possible.
Radiation therapy applied after the onset of severe motor paralysis or central pain development previously showed lower efficacy than when it was applied while the symptoms were mild. Prophylactic radiation therapy for bone metastases has been reported to provide durable tumor control (3-5). In addition, direct decompressive surgical resection can improve symptoms following spinal cord injury caused by spinal metastasis (6). Thus, early intervention is essential to prevent irreversible neurological disorders associated with spinal metastases.
However, in clinical practice, interventions are typically first considered when patients with spinal metastases present with severe pain and/or motor paralysis. Such delayed application of interventions for spinal metastases may be due to the patients’ lack of awareness of their symptoms, as well as the lack of knowledge of the medical staff. Radiation therapy and surgical decompression certainly have considerable benefits for symptom management and tumor control, but they also have some complications. Radiation therapy can cause bone necrosis, which may worsen spinal instability and fragility in patients with spinal metastases (7). Surgical decompression may be linked to severe postoperative complications, such as bleeding, pneumonia, and heart failure, and may demonstrate limited efficacy in symptom management (8). Therefore, it is unrealistic that every case of spinal metastasis should be treated with radiation therapy and/or decompressive surgery at the time of detection. Thus, there is a need for a means of inferring when and in which patients such interventions are required.
In this study, we explored the imaging characteristics of spinal metastases that may lead to the development of neurological disorders to identify patients with spinal metastases for whom early intervention would have a favorable benefit-to-risk ratio. We present the following article in accordance with the STARD reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-21-3909/rc).
Methods
Participants
The present study was conducted in accordance with the ethical standards of the Declaration of Helsinki (as revised in 2013) and the ethical guidelines for medical and health research presented by the Ministry of Health, Labour, and Welfare in Japan. This study was approved by the Ethics Committee of The University of Tokyo Hospital [approval No. 3905-(5)] and opt-out consents were obtained from all participants. We retrospectively enrolled 161 patients, who were treated by our palliative care team from September 2012 to July 2019 [89 males; mean age 64.2±13.1 years (mean ± standard deviation); Table 1]. All patients had solid tumors, excluding non-epithelial solid malignancies, such as sarcoma, with spinal bone metastases (Table 2). Patients with a history of treatment for spinal bone metastases were excluded. Regardless of whether the patients received any analgesics, we enrolled them. The clinical symptoms corresponded with the analyzed levels and laterality, which were confirmed by experienced pain physicians. Motor paralysis was defined as muscle weakness of the lower limb(s) with or without bladder and rectal disturbance, of which any causes other than spinal metastases could be ruled out. Neuropathic pain was assessed based on a grading system for the diagnosis of neuropathic pain from the International Association for the Study of Pain Neuropathic pain Special Interest Group (9). The patients diagnosed as neuropathic pain in this study suffered from spinal nerve root symptoms, of which the pain distribution topographically consisted with the peripheral innervation territory. Local nociceptive pain was defined as pain present only in the area of spinal metastases without any radiating pain distribution (e.g., back pain). Any pain intensity of neuropathic pain and local nociceptive pain was permitted.
Table 1
| Characteristics | Symptomatic group (at the onset, n=63) | Asymptomatic group (n=98) | P value |
|---|---|---|---|
| Sex | |||
| Men | 36 | 53 | 0.75 |
| Women | 27 | 45 | |
| Age (mean), years | 61.87 | 65.7 | 0.12 |
| Classification of symptoms | |||
| At the onset (men/women) | |||
| Motor paralysis | 15 (7/8) | – | – |
| Neuropathic pain | 28 (15/13) | – | – |
| Local nociceptive pain | 22 (14/8) | – | – |
| Before the onset (men/women) | |||
| Motor paralysis | 9 (4/5) | – | – |
| Neuropathic pain | 11 (6/5) | – | – |
| Local nociceptive pain | 7 (5/2) | – | – |
| Number of images | 411 | 666 | |
| At the onset | 340 | ||
| Motor paralysis | 79 | – | – |
| Neuropathic pain | 176 | – | – |
| Local nociceptive pain | 98 | – | – |
| Before the onset | 71 | ||
| Motor paralysis | 23 | – | – |
| Neuropathic pain | 34 | – | – |
| Local nociceptive pain | 20 | – | – |
Table 2
| Primary site | Symptomatic group (onset) | Asymptomatic group | P value | ||
|---|---|---|---|---|---|
| Motor paralysis | Neuropathic pain | Local nociceptive pain | |||
| Central nerve system | 0 | 0 | 1 | 0 | 0.40 |
| Oral cavity and pharynx | 2 | 3 | 1 | 8 | <1.0 |
| Lung | 0 | 3 | 4 | 26 | 0.016 |
| Breast | 2 | 3 | 2 | 13 | 0.81 |
| Gastrointestinal | 4 | 7 | 6 | 20 | 0.45 |
| Hepatobiliary | 0 | 7 | 1 | 13 | <1.0 |
| Urinary, bladder, prostate | 4 | 4 | 3 | 12 | 0.49 |
| Uterine, ovary | 1 | 0 | 1 | 5 | 0.70 |
| Bone | 1 | 0 | 1 | 1 | 0.56 |
| Skin | 1 | 1 | 1 | 0 | 0.06 |
| Unknown | 0 | 0 | 1 | 0 | 0.40 |
We divided patients into those with and those without symptoms, including motor paralysis of lower limbs, neuropathic pain, and/or local nociceptive pain due to spinal metastases (Table 1). These symptoms were evaluated and specified as sequelae due to spinal metastases by our experienced palliative care physicians. Bone metastases are classified as osteolytic, osteoblastic, or mixed, according to the primary mechanism of interference with normal bone remodeling. More than 70% of spinal metastases are osteolytic, 8% are osteoblastic, and 21% are mixed (10). We classified our participants into two types (i.e., osteoblastic and osteolytic). Patients with mixed metastases were included in the osteolytic group (Table S1).
Spinal metastases of the study participants were appropriately confirmed with clinical diagnostic imaging by our non-experimenter radiologists. We analyzed computed tomography (CT) axial cross-sectional images of the spine, ranging from the 3rd cervical vertebral level (C3) to the 10th thoracic vertebral level (Th10). We focused on these spinal levels because the configurations of C1 and C2 are quite different from those of other spinal vertebrae, which are similar in shape and because, up to Th10, the spinal cord is definitely present (2,11). Symptoms of cauda equina syndrome caused by spinal metastases below Th10 are sometimes indistinguishable from those of pre-existing lumbar spinal canal stenosis and neuropathy, such as chemotherapy-induced peripheral polyneuropathy.
For patients without any symptoms of spinal metastases (asymptomatic group, n=98), we collected a total of 666 slices of spinal CT images when we started palliative intervention for symptoms unrelated to spinal metastases (e.g., visceral pain, nausea, and dyspnea). On the other hand, for those with any symptoms (n=63), we collected 340 slices of spinal CT images immediately upon starting palliative intervention. Among the symptomatic patients, we could collect 71 slices of the spinal CT images that were recorded before the onset of any symptoms due to spinal metastases (4.1±3.9 months backward in time) in 26 patients.
We categorized spinal metastases-related symptoms into three types: the first involved motor paralysis of the lower limbs; the second involved neuropathic pain in the corresponding dermatome of the spinal root nerve at the level of the spinal metastases; and the third was local nociceptive pain (Figure 1). According to this categorization, the “motor paralysis” type consisted of 15 patients with 79 CT image slices; the “neuropathic pain” type consisted of 28 patients with 176 slices; and the “local nociceptive pain” type consisted of 22 patients and 98 slices. We conducted image analyses with respect to each type of spinal metastases-related symptom. Furthermore, we analyzed 9 patients and 23 slices for motor paralysis, 11 patients and 34 slices for neuropathic pain, and 7 patients and 20 slices for local nociceptive pain using imaged obtained before the onset of these symptoms.
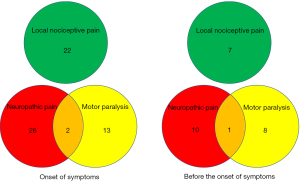
Image analysis
We used the image analyzer software, WinROOF2018© (Mitani, Tokyo, Japan), for image analysis.
Each CT axial cross-sectional slice was divided into 400 cells (20 in length and 20 in width), arranged in a square reticular pattern (Figure 2). The outer frame of the square-grid was configured as follows: the midpoint of the top side was matched with the top surface of the vertebral body, and the length of one side was set between the bilateral edges of the transverse processes. The grid included as much of the spinous process as possible, but it was acceptable if some tip areas of the spinous process were not included within the range of the grid. Using this condition, the center of the grid matched the top of the spinal canal in most slices. Using the software, the area of spinal metastasis was automatically marked based on low density, which indicated osteolytic change, and then we visually checked the marked area and corrected it manually if necessary. We also manually marked the area of bone metastasis with high density, which indicates osteosclerotic change. When the proportion of the metastasis area in the respective cells of the grid was 20% or more, we counted these cells as having tumor metastasis present. Thus, dichotomous measures (i.e., presence or absence) of tumor metastasis were assessed for all of the 400 cells in one slice.

Laterality of spinal metastasis images reportedly does not demonstrate any differences in features (12). Considering this notion, the right-half image of each slice was flipped horizontally; thus, we duplicated the left-half images of each slice. Therefore, we used both the left and right halves of the information on spinal metastases. We finally created tumor distribution maps for each symptom-type group and for the asymptomatic group. We superimposed all slices of the patient group, and then, for the respective cells, we indicated the proportion (0–100%) of slices with tumor present relative to the total number of superimposed slices.
Statistical analysis
The statistical significance of the differences between each symptom-type group and the asymptomatic group was determined using the Mann-Whitney test.
We compared the respective cells of the spinal tumor distribution map for each symptom-type group to the asymptomatic group using the χ-square test with P values and odds ratio. We replicated these procedures to compare data of the asymptomatic group with data of the symptom-type groups obtained before the onset of the respective symptoms. We aimed to determine regions on the tumor distribution maps, obtained from images taken before symptom onset, where tumor progression seemed to infer the emergence of the symptoms or where spinal metastases were observed but were unlikely to lead to symptom development. In addition, each symptom was classified as osteoblastic or osteolytic, and data on the distribution of osteoblastic and osteolytic lesions according to symptoms were compared.
Results
The age and sex of the asymptomatic group were comparable to those of the symptomatic group at the time of onset (Table 1). In the symptomatic group, the most common primary cancer sites were the urinary tract and gastrointestinal tract for patients with motor paralysis; the gastrointestinal tract and hepatobiliary tract for those with neuropathic pain; and the gastrointestinal tract for those with local nociceptive pain. On the other hand, in the asymptomatic group, the respiratory system was the most common site (Table 2). The number of patients and slices with osteolytic lesions were larger than those with osteoblastic lesions. In contrast, osteoblastic lesions were more common in the asymptomatic group and the local nociceptive pain group before onset (Table S1).
Symptomatic patients with motor paralysis demonstrated that spinal metastases significantly more often occupied areas in and around the spinal canal as well as areas around the pedicle than asymptomatic patients (Figure 3). The osteolytic type was more common in the spinal canal (Figure S1). Before the onset of the motor paralysis, spinal metastases were observed in both areas near the pedicle and the most posterior areas of the vertebral body (Figure 3). There were some significant regions between the osteolytic and osteoblastic types at and before the onset of motor paralysis (Figure S1). However, these regions were inconsistent throughout the observation period, which could specifically indicate symptom onset.

The “neuropathic pain” type patients demonstrated the spread of spinal metastases along the pedicle and circumferentially around the spinal canal at and before onset of symptoms (Figure 3). Osteolytic regions were more commonly observed in these areas (Figure S1) at the onset, but not before the onset of symptoms.
Local nociceptive pain in the metastatic spine frequently occurred in areas around the center of the vertebral body, which were virtually the same areas in which lesions were present before the onset of this pain (Figure 3). The osteoblastic regions lesioned into the inter-circumference of the vertebral body at the onset of symptoms. In contrast, the osteolytic regions were observed on the lateral surface of the vertebral body (Figure S1).
In the asymptomatic group, only a few regions demonstrated significant differences between the osteolytic and osteoblastic types (Figure S1).
Discussion
We here explored the imaging characteristics of spinal metastases in patients with specific spinal metastasis-related symptoms (motor paralysis, neuropathic pain, and local nociceptive pain) compared with those in patients without any such symptoms. In patients with motor paralysis, spinal metastasis lesions were present in and around the spinal canal and spread to regions around the pedicle of the spinal arch at the time of the symptom onset. Lesions in regions more lateral to the pedicle of the spinal arch and in the most posterior part of the vertebral body were found to be involved in the later development of motor paralysis. Osteolytic lesions are more common in these regions, and, in general, are more likely to extend outside of the bone and invade the spinal canal. We also found lesions in regions related to neuropathic pain in the corresponding dermatome of the spinal root nerve at the level of the spinal metastases. In addition to motor paralysis, osteolytic lesions are commonly observed in these regions. Bone destruction and collapse by osteolytic lesions may lead to bone deformity and subsequent compression of the nerve roots. As osteolytic lesions are generally more prone to extraskeletal extension, the lesions might extend extraskeletal from the intervertebral foramen and subsequently infiltrate the nerve roots directly. Moreover, we found lesions before the onset and at the time of symptom emergence that was related to local nociceptive pain. Spinal metastases were observed in these regions during the asymptomatic period, suggesting that these regions may infer the emergence of symptoms after a period of several months.
Cancer that spreads to the spine can compress the spinal cord and the nearby spinal structures, and, if left untreated, this can lead to pain, motor paralysis, and incontinence. Some inferable scoring systems for patients with cancer with spinal metastases (13-15) are useful for inferring their survival prognosis. Predictors of survival in patients with spinal metastases, but the presence of spinal metastases-related symptoms is not included. These inferable systems are usually referenced when considering the indication for spinal metastasis surgeries to improve motor paralysis, pain refractory to several lines of pharmacotherapy, and ADLs (16). Because of the limited survival prognosis and impaired general condition due to advanced cancer status, spinal metastasis surgeries seem to be highly selective in clinical settings. As an alternative to spinal surgeries, radiotherapy and anti-cancer pharmacotherapy can be applied to reduce the pressure on the spinal cord when spinal metastases occur. However, none of these studies have yet demonstrated sufficient evidence to support the recovery of spinal metastases-related symptoms (17). Once symptoms appear in patients with spinal metastases, their health-related QOL can be substantially impaired until the end of life. In light of this, early interventions that can prevent the onset of spinal metastases-related symptoms should be considered for cases with asymptomatic or minimally symptomatic spine metastases. However, to the best of our knowledge, there are no inferable systems that can infer the onset of spinal metastases-symptoms.
In this study, we identified some regions in the spine in which spinal metastasis lesions during the asymptomatic stage could strongly infer the emergence of spinal metastases-related symptoms after a period of several months. We separately identified the regions inferable for motor paralysis, neuropathic pain, and local nociceptive pain.
In patients complaining of local nociceptive pain, metastatic regions were found around the center of the vertebral body. This finding is consistent with a previous notion that the location of the tumor in the central third of the axial direction is a risk factor for vertebral collapse (18). However, we also found inferable regions for local nociceptive pain in the posterior third, but do not exist in the anterior third of the vertebral body. The location and size of the tumor in the spine have been cited as factors related to spinal instability and destruction. A disproportionate anteroposterior distribution of metastases within the vertebral body might be specifically predisposed to cause spinal instability and destruction. Radiation and possibly spinal surgery for asymptomatic or minimally symptomatic spinal metastases have been shown to significantly reduce the risk of developing such skeletal-related events (5,19).
As most symptomatic spinal metastases are immediately treated with radiation therapy or decompressive surgery when detected, our findings of inferable regions for local nociceptive pain in patients with spinal metastases may be of little benefit. In the case of imaging findings that may indicate the onset of symptoms, careful monitoring should be performed, and treatment should be initiated as early as possible according to the expansion of the lesion to prevent the onset of serious symptoms.
Motor paralysis, in conjunction with neuropathic pain due to spinal lesions, can profoundly impair health-related QOL. We found that the presence of metastatic lesions in regions outside the pedicle of the spinal arch in the asymptomatic phase is associated with the emergence of these symptoms, which can strongly infer later symptom onset. Metastases around the pedicle may affect the spinal nerve and subsequently cause neuropathic pain. Spinal metastases in the most posterior region of the vertebral body, facing the spinal canal, are considered to indicate a risk of spinal cord compression because these metastases are associated with instability of spinal alignment (20,21). However, our findings suggest that patients with metastatic lesions in the regions around the pedicle require closer monitoring for symptom onset than those with lesions in the posterior third of the vertebral body. In cases of spinal metastasis-related spinal cord injury, surgery is reportedly superior to other interventions in terms of improving the QOL and medical costs (22). Because surgery is generally more invasive than other interventions, the indication should be considered more carefully to balance its risks and benefits. In general, surgery is not indicated for asymptomatic spinal metastases. Early identification of the risk group for spinal cord injury, when their general condition is not severely deteriorated, may be beneficial in expanding the indication for surgery for spinal metastases and in completely preventing spinal cord injury. As the prevalence of spinal cord compression from metastatic tumors has been historically confirmed to be very low (15%) (23), our imaging findings might be useful in identifying the group at high risk of spinal cord injury during the asymptomatic period.
There are several limitations in this study. First, this was a retrospective study based on medical records, and it was difficult to evaluate patient symptoms that were not described precisely in the medical records. Second, in this case, we discuss bone pain; however, soft tissue pain may also be included because some tumors extend beyond the bone. There was no significant difference in the local nociceptive pain between the two origins. Third, we used CT images because CT images are widely used and allow a simple and rapid examination, with fewer limitations than MRI. Our identified regions might be useful for a wide range of oncology services. However, the inter-osseous type of bone metastases can only be detected by MRI (24). Finally, the number of participants was limited. In particular, there were few patients demonstrating motor paralysis and neuropathic pain; therefore, we did not conduct a confirmatory study of the inferable regions using other patient cohorts. Because of the limited numbers of the patients, we also did not consider the concurrent use of denosumab and the primary cancer sites. In future studies, larger patient cohorts should be analyzed, and the accuracy of the inferable regions should be validated.
We show that the presence of metastatic lesions in particular regions of the spine can infer the emergence of spinal metastasis-related symptoms within a few months, with high specificity. This provides a screening tool for early therapeutic intervention to prevent neurological disorder.
Acknowledgments
Funding: This study was funded by Japanese Society for the Promotion of Science (grant numbers: JSPS KAKENHI, 19H03749).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-21-3909/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-21-3909/dss
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-21-3909/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-21-3909/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The present study was conducted in accordance with the ethical standards of the Declaration of Helsinki (as revised in 2013) and the ethical guidelines for medical and health research presented by the Ministry of Health, Labour, and Welfare in Japan, and was approved by the Ethics Committee of The University of Tokyo Hospital [approval No. 3905-(5)] and the opt-out consents were obtained from all participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Finnerup NB, Norrbrink C, Trok K, et al. Phenotypes and predictors of pain following traumatic spinal cord injury: a prospective study. J Pain 2014;15:40-8. [Crossref] [PubMed]
- Zeilig G, Enosh S, Rubin-Asher D, et al. The nature and course of sensory changes following spinal cord injury: predictive properties and implications on the mechanism of central pain. Brain 2012;135:418-30. [Crossref] [PubMed]
- van Oorschot B, Rades D, Schulze W, et al. Palliative radiotherapy--new approaches. Semin Oncol 2011;38:443-9. [Crossref] [PubMed]
- Ruckdeschel JC. Early detection and treatment of spinal cord compression. Oncology (Williston Park) 2005;19:81-6; discussion 86, 89-92.
- Shulman RM, Meyer JE, Li T, et al. External beam radiation therapy (EBRT) for asymptomatic bone metastases in patients with solid tumors reduces the risk of skeletal-related events (SREs). Ann Palliat Med 2019;8:159-67. [Crossref] [PubMed]
- Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet 2005;366:643-8. [Crossref] [PubMed]
- Bartlow CM, Mann KA, Damron TA, et al. Limited field radiation therapy results in decreased bone fracture toughness in a murine model. PLoS One 2018;13:e0204928. [Crossref] [PubMed]
- Feiz-Erfan I, Rhines LD, Weinberg JS. The role of surgery in the management of metastatic spinal tumors. Semin Oncol 2008;35:108-17. [Crossref] [PubMed]
- Haanpää M, Attal N, Backonja M, et al. NeuPSIG guidelines on neuropathic pain assessment. Pain 2011;152:14-27. [Crossref] [PubMed]
- Tsukamoto S, Kido A, Tanaka Y, et al. Current Overview of Treatment for Metastatic Bone Disease. Curr Oncol 2021;28:3347-72. [Crossref] [PubMed]
- Finnerup NB, Johannesen IL, Fuglsang-Frederiksen A, et al. Sensory function in spinal cord injury patients with and without central pain. Brain 2003;126:57-70. [Crossref] [PubMed]
- Wang C, Shen Y. Study on the distribution features of bone metastases in prostate cancer. Nucl Med Commun 2012;33:379-83. [Crossref] [PubMed]
- Tokuhashi Y, Ajiro Y, Umezawa N. Outcome of treatment for spinal metastases using scoring system for preoperative evaluation of prognosis. Spine (Phila Pa 1976) 2009;34:69-73. [Crossref] [PubMed]
- Leithner A, Radl R, Gruber G, et al. Predictive value of seven preoperative prognostic scoring systems for spinal metastases. Eur Spine J 2008;17:1488-95. [Crossref] [PubMed]
- Katagiri H, Okada R, Takagi T, et al. New prognostic factors and scoring system for patients with skeletal metastasis. Cancer Med 2014;3:1359-67. [Crossref] [PubMed]
- Molina CA, Gokaslan ZL, Sciubba DM. A systematic review of the current role of minimally invasive spine surgery in the management of metastatic spine disease. Int J Surg Oncol 2011;2011:598148. [Crossref] [PubMed]
- George R, Jeba J, Ramkumar G, et al. Interventions for the treatment of metastatic extradural spinal cord compression in adults. Cochrane Database Syst Rev 2015;CD006716. [Crossref] [PubMed]
- Krishnaney AA, Steinmetz MP, Benzel EC. Biomechanics of metastatic spine cancer. Neurosurg Clin N Am 2004;15:375-80. [Crossref] [PubMed]
- Tomita K, Kawahara N, Kobayashi T, et al. Surgical strategy for spinal metastases. Spine (Phila Pa 1976) 2001;26:298-306. [Crossref] [PubMed]
- Prasad D, Schiff D. Malignant spinal-cord compression. Lancet Oncol 2005;6:15-24. [Crossref] [PubMed]
- Mauch JT, Carr CM, Cloft H, et al. Review of the Imaging Features of Benign Osteoporotic and Malignant Vertebral Compression Fractures. AJNR Am J Neuroradiol 2018;39:1584-92. [Crossref] [PubMed]
- Regine WF, Tibbs PA, Young A, et al. Metastatic spinal cord compression: a randomized trial of direct decompressive surgical resection plus radiotherapy vs. radiotherapy alone. Int J Radiat Oncol Biol Phys 2003;57:S125. [Crossref]
- Robson P. Metastatic spinal cord compression: a rare but important complication of cancer. Clin Med (Lond) 2014;14:542-5. [Crossref] [PubMed]
- Yamaguchi T. Intertrabecular vertebral metastases: metastases only detectable on MR imaging. Semin Musculoskelet Radiol 2001;5:171-5. [Crossref] [PubMed]


