
The association between immunosuppressants use and COVID-19 adverse outcomes: national COVID-19 cohort in South Korea
Introduction
In December 2019, an outbreak of a novel coronavirus was reported in Wuhan, China, which was later named the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by the Coronavirus Study Group of the International Committee on Taxonomy of Viruses (1,2). The SARS-CoV-2 virus rapidly spread around the world, causing the COVID-19 disease in infected patients; on March 12, 2020, the World Health Organization (WHO) officially declared COVID-19 a pandemic (3).
As the scientific community raced to find possible active agents for treatment and cure of COVID-19, supportive care therapies were developed and tested (3,4). Immunosuppressants, specifically corticosteroids, were employed, as it could help COVID-19 patients by mitigating cytokine storms (5). However, there are concerns that it can worsen viral shedding and, therefore, increase mortality due to COVID-19 (6).
The current WHO clinical guidelines, as of November 2021, provides a strong recommendation for corticosteroids use in severe and critical patients with COVID-19 infection (7). However, in patients with mild to moderate COVID-19 infections who do not require oxygen support, corticosteroid use was associated with a trend towards higher mortality, although not statistically significant in the RECOVERY trial (17.8% vs. 14.0%; RR =1.19; 95% CI: 0.92–1.55) (8). As a result, there is uncertainty of the effect of immunosuppression, including corticosteroids, started before COVID-19 infection, on COVID-19 outcomes since patients on immunosuppression could have a wide spectrum of COVID-19 disease presentations, from asymptomatic to critical COVID-19 infections. It is possible that patients with non-severe COVID-19 infections could receive harm from baseline corticosteroids use.
The aim of this study was to investigate the relationship between immunosuppressants use, including corticosteroids, and COVID-19 patient outcomes. We present the following article in accordance with the STROBE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-21-3465/rc).
Methods
Data source and study population
The Health Insurance Review and Assessment Service (HIRA) of South Korea is the sole nationwide government agency that operates a fee-for-service reimbursement system, which covers 98% of the Korean population. Its administrative claims database includes the beneficiary’s sociodemographic characteristics, healthcare utilization history, diagnosis results (International Classification of Diseases 10th Revision, ICD-10), as well as prescription from both inpatient and outpatient settings. On March 27, 2020, the #OpenData4COVID19 project by the Ministry of Health and Welfare (MoHW) of Korea released a patient-level, deidentified COVID-19 data based on the HIRA insurance claims database, which is the first nationwide dataset of COVID-19 patients. The HIRA COVID-19 database included data for all individuals who received a reverse transcription-polymerase chain reaction (RT-PCR) test for COVID-19 as of May 15, 2020, which was linked to their claims data for the previous 3 years.
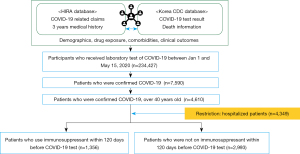
The HIRA data consisted of 234,427 consecutive individuals who were tested for SARS-CoV-2 between January 1, 2020 and May 15, 2020. In total, 7,590 were identified as positive for COVID-19, designated by the coding in the #OpenData4COVID19 project (Table S1). Among them, 4,610 individuals were aged 40 years or older. We excluded those who were less than 40 years old, as it is less likely that these younger individuals would experience severe COVID-19 outcomes. For precise outcome measurements, we further restricted the analysis to individuals who were hospitalized for COVID-19, resulting in 4,349 individuals in our study cohort (Figure 1). The cohort entry date was defined as the admission date of COVID-19 hospitalization. In South Korea, individuals who were confirmed to be positive for COVID-19 during this time interval required hospitalization until full recovery, defined by the cessation of fever without medication use and two negative test results within 24 hours (9). However, there were a small number of confirmed COVID-19 patients who were not hospitalized due to the temporal shortage of health facilities.
This study was approved by the Human Investigation Review Board of Public Institutional Bioethics Committee designated by the MOHW, which waived the requirement of informed consent due to retrospective study design and anonymity of the HIRA database (IRB #P01-2020-1262-001). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Endpoints
Our primary endpoint was a composite of all-cause death, intensive care unit (ICU) admission, and mechanical ventilation use. As secondary endpoints, we evaluated the individual components of the primary composite endpoint. In-hospital ICD-10 diagnostic codes and the national procedure codes were used to define the outcomes (Table S1). We measured these study outcomes between the cohort entry date through the end of follow-up (discharge, or end of study).
Exposures
We classified individuals who had inpatient and outpatient prescription records of immunosuppressants at least once within 120 days prior to the cohort entry (ascertainment window: −120 to 0 d) as immunosuppressants users. Other individuals were defined as non-users. Among immunosuppressants users, corticosteroids users were assessed as a separate exposure group and compared to the non-immunosuppressants-users in sensitivity analysis. Our definition follows an intention-to-treat approach. Immunosuppressants were identified by the anatomical therapeutic chemical classification codes (ATC) (Table S1).
Statistical analysis
We described baseline characteristics of immunosuppressants users and non-users as counts with percentages (for categorical variables) and means with standard deviations (for continuous variables). We calculated the absolute standard difference (aSD) to measure distributional imbalances between two groups for each variable. Empirically, aSD ≤0.1 is preferred for balance between groups.
We conducted outcome analysis by estimating odds ratios (ORs) using a logistic regression analysis where each individual was weighted by the inverse probability of treatment weight (IPTW) (10,11) To be specific, we estimated the propensity score (PS), the probability for an individual to receive immunosuppressants, using logistic regression, which included age in years, square of age, sex, and type of health insurance at cohort entry, as well as 20 pre-exposure comorbidities and 12 pre-exposure co-medications (Appendix 1) as explanatory variables. We defined comorbidity variables using diagnostic codes and co-medications (Table S1). Then, the IPTW, 1/PS to immunosuppressants users and 1/(1-PS) to non-users, was assigned to each individual. The effect of immunosuppressants use on primary and secondary endpoints was then estimated using weighted univariable logistic regression. We reported the estimated OR and the 95% confidence intervals (CI). In addition, for comparison purposes, we fitted unweighted univariate logistic regression models and unweighted multivariate logistic regression models, adjusting for age, sex, insurance type, history of diabetes and history of hypertension.
We conducted a subgroup analysis for the risk of the primary endpoint. We considered stratifications by the following: (I) sex; (II) age, classified into groups with age <65 and ≥65 years; (III) the history of autoimmune disease, cancer and HIV, and (IV) the history of autoimmune disease. For each subgroup analysis, we conducted a trivariable IPTW-weighted logistic regression that includes the exposure (immunosuppressants use), stratification variable and the interaction of those two. We obtained the P value of the interaction term (P for interaction) for each stratification to examine the effect modification.
We checked the sensitivity of results against the scope of working definitions. First, we redefined the study population by those who were aged ≥40 years and confirmed positive, which relaxed the restriction of hospitalization. Second, we also considered an exclusion for individuals who used immunosuppressants for a short period but stopped prior to hospitalization, and thus we narrowed down the window of ascertaining exposure from 120 to 90 days. Lastly, we narrowed down the definition of the exposure group from immunosuppressants users to corticosteroids users. For each change of the settings, we repeated our main analysis.
To examine the sensitivity of results against the selection of statistical tools, the following alternative approaches were considered. First, we excluded subjects with extreme PS values, that is, PS <0.025 or PS >0.975 (IPTW with trimming). Second, we additionally included the fitted PSs to other covariates in our unweighted multivariable logistic regression model (outcome adjustment model). Third, we used the standardized mortality ratio (SMR) weight, defined as 1 for immunosuppressants users and PS/(1-PS) for non-users, in place of IPTW in our main model (SMR weighting). Finally, we considered a PS matching approach (PS matching). All statistical analyses were conducted using R 3.5.2. P values less than 0.05 were considered statistically significant.
Results
A total of 4,349 patients were included in the main analysis, for which 1,356 (31%) used immunosuppressants within 120 days of hospitalization for COVID-19. Users of immunosuppressants were older, and a larger proportion had hyperlipidemia and hypertension. A larger proportion of immunosuppressants users also used acetaminophen, systematic antibiotics, and NSAIDs (Table 1).
Table 1
| Characteristic | Before IPTW | After IPTW§ | |||||
|---|---|---|---|---|---|---|---|
| User* (n=1,356) | Non-user (n=2,993) | aSD | User* (n=4,349) | Non-user (n=4,360) | aSD | ||
| Age (years; mean ± SD) | 61.4±12.0 | 59.7±12.7 | 0.14 | 60.2±12.7 | 60.2±12.5 | 0.00 | |
| 40–49 | 229 (16.9) | 679 (22.7) | 0.15 | 954 (21.9) | 901 (20.7) | 0.03 | |
| 50–59 | 424 (31.3) | 983 (32.8) | 0.03 | 1,406 (32.3) | 1,404 (32.2) | 0.00 | |
| 60–69 | 371 (27.4) | 692 (23.1) | 0.10 | 1,010 (23.2) | 1,068 (24.5) | 0.03 | |
| 70–79 | 205 (15.1) | 366 (12.2) | 0.08 | 563 (13.0) | 595 (13.6) | 0.02 | |
| 80–89 | 113 (8.3) | 220 (7.4) | 0.04 | 339 (7.8) | 319 (7.3) | 0.02 | |
| 90+ | 14 (1.0) | 53 (1.8) | 0.06 | 77 (1.8) | 72 (1.7) | 0.01 | |
| Sex | |||||||
| Male | 483 (35.6) | 1,126 (37.6) | 0.04 | 1,591 (36.6) | 1,600 (36.7) | 0.00 | |
| Female | 873 (64.4) | 1,867 (62.4) | 0.04 | 2,759 (63.4) | 2,760 (63.3) | 0.00 | |
| Health insurance type | |||||||
| Medical insurance | 1,192 (87.9) | 2,639 (88.2) | 0.01 | 3,846 (88.4) | 3,848 (88.3) | 0.01 | |
| Medical aid | 164 (12.1) | 354 (11.8) | 0.01 | 503 (11.6) | 512 (11.7) | 0.01 | |
| Comorbidities | |||||||
| Arrhythmias | 57 (4.2) | 62 (2.1) | 0.12 | 118 (2.7) | 117 (2.7) | 0.00 | |
| Asthma | 208 (15.3) | 239 (8.0) | 0.23 | 441 (10.1) | 450 (10.3) | 0.01 | |
| Atrial fibrillation | 32 (2.4) | 43 (1.4) | 0.07 | 74 (1.7) | 79 (1.8) | 0.01 | |
| Autoimmune disease | 177 (13.1) | 75 (2.5) | 0.40 | 255 (5.9) | 267 (6.1) | 0.01 | |
| Chronic lung disease | 525 (38.7) | 901 (30.1) | 0.18 | 1,459 (33.6) | 1,457 (33.4) | 0.00 | |
| Coronary artery disease | 134 (9.9) | 192 (6.4) | 0.13 | 331 (7.6) | 336 (7.7) | 0.00 | |
| Dementia | 80 (5.9) | 234 (7.8) | 0.08 | 321 (7.4) | 319 (7.3) | 0.00 | |
| Diabetes mellitus | 275 (20.3) | 562 (18.8) | 0.04 | 853 (19.6) | 844 (19.4) | 0.01 | |
| Heart failure | 71 (5.2) | 117 (3.9) | 0.06 | 199 (4.6) | 181 (4.1) | 0.02 | |
| Hyperlipidemia | 516 (38.1) | 910 (30.4) | 0.16 | 1,432 (32.9) | 1,433 (32.9) | 0.00 | |
| Hypertension | 407 (30.0) | 742 (24.8) | 0.12 | 1,133 (26.1) | 1,142 (26.2) | 0.00 | |
| Immunosuppression# | 159 (11.7) | 219 (7.3) | 0.15 | 389 (8.9) | 383 (8.8) | 0.01 | |
| Kidney disease | 22 (1.6) | 37 (1.2) | 0.03 | 51 (1.2) | 55 (1.3) | 0.01 | |
| Liver disease | 67 (4.9) | 129 (4.3) | 0.03 | 196 (4.5) | 192 (4.4) | 0.00 | |
| Other cerebrovascular diseases | 98 (7.2) | 150 (5.0) | 0.09 | 238 (5.5) | 248 (5.7) | 0.01 | |
| Peripheral vascular disease | 148 (10.9) | 246 (8.2) | 0.09 | 385 (8.9) | 390 (8.9) | 0.00 | |
| Pneumonia including tuberculosis | 129 (9.5) | 182 (6.1) | 0.13 | 311 (7.1) | 308 (7.1) | 0.00 | |
| Psychiatric disorders | 408 (30.1) | 706 (23.6) | 0.15 | 1,082 (24.9) | 1,111 (25.5) | 0.01 | |
| Stroke or TIA | 105 (7.7) | 198 (6.6) | 0.04 | 287 (6.6) | 299 (6.9) | 0.01 | |
| Thromboembolism | 71 (5.2) | 161 (5.4) | 0.01 | 225 (5.2) | 229 (5.3) | 0.00 | |
| Medications | |||||||
| Acetaminophen | 409 (30.2) | 539 (18.0) | 0.29 | 960 (22.1) | 960 (22.0) | 0.00 | |
| Antibacterials | 628 (46.3) | 994 (33.2) | 0.27 | 1,627 (37.4) | 1,641 (37.6) | 0.00 | |
| Anticoagulants | 50 (3.7) | 58 (1.9) | 0.11 | 113 (2.6) | 112 (2.6) | 0.00 | |
| Antidementia | 100 (7.4) | 248 (8.3) | 0.03 | 334 (7.7) | 341 (7.8) | 0.01 | |
| Antidepressants | 159 (11.7) | 255 (8.5) | 0.11 | 429 (9.9) | 416 (9.5) | 0.01 | |
| Antidiabetics | 199 (14.7) | 415 (13.9) | 0.02 | 620 (14.3) | 626 (14.4) | 0.00 | |
| Antiplatelets | 220 (16.2) | 359 (12.0) | 0.12 | 566 (13.0) | 581 (13.3) | 0.01 | |
| Antipsychotics | 186 (13.7) | 422 (14.1) | 0.01 | 573 (13.2) | 607 (13.9) | 0.02 | |
| Antivirals | 56 (4.1) | 82 (2.7) | 0.08 | 136 (3.1) | 137 (3.1) | 0.00 | |
| Anxiolytics | 264 (19.5) | 439 (14.7) | 0.13 | 698 (16.1) | 711 (16.3) | 0.01 | |
| Lipid lowering agents including statin | 392 (28.9) | 673 (22.5) | 0.15 | 1,064 (24.5) | 1,083 (24.8) | 0.01 | |
| NSAIDs | 844 (62.2) | 1,294 (43.2) | 0.39 | 2,138 (49.2) | 2,152 (49.4) | 0.00 | |
Mean and standard deviation were reported for continuous variables. Frequency and percentage were reported for categorical variables. Users: immunosuppressants within 120 days. *, patients prescribed within 120 days prior to cohort entry were regarded as immunosuppressants users, and otherwise patients were defined as non-users; §, weighted cohort using the IPTW; #, immunosuppression includes HIV, history of organ transplant, transplant rejection, noninfectious enteritis and colitis, ulcerative colitis, and Crohn’s disease. IPTW, inverse probability of treatment weighting; aSD, absolute standardized difference; TIA, transient cerebral ischemic attack; NSAIDs, nonsteroidal anti-inflammatory drugs.
Patients who used immunosuppressants were at increased odds of the primary endpoint of all-cause death, ICU admission and mechanical ventilation use (IPTW OR =1.32; 95% CI: 1.06–1.63). When assessing the component outcomes individually, immunosuppressants users were at higher odds of all-cause mortality—IPTW OR =1.63; 95% CI: 1.21–2.26 (Table 2).
Table 2
| Outcomes | Cumulative incidence (%) | Unadjusted* | Adjusted§ | IPTW adjusted# | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-user | User | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | ||||
| Composite primary outcome | 311 (10.4) | 185 (13.6) | 1.36 (1.12–1.66) | 0.002 | 1.31 (1.07–1.60) | 0.01 | 1.32 (1.06–1.63) | 0.01 | |||
| All-cause death | 126 (4.2) | 91 (6.7) | 1.64 (1.24–2.16) | 0.001 | 1.68 (1.23–2.30) | 0.001 | 1.63 (1.21–2.26) | <0.01 | |||
| Mechanical ventilation use | 72 (2.4) | 51 (3.8) | 1.59 (1.10–2.28) | 0.01 | 1.48 (1.02–2.14) | 0.004 | 1.12 (0.76–1.65) | 0.57 | |||
| ICU admission | 212 (7.1) | 118 (8.7) | 1.25 (0.99–1.58) | 0.06 | 1.22 (0.96–1.54) | 0.10 | 1.11 (0.87–1.44) | 0.38 | |||
*, unweighted univariable logistic regression model; §, unweighted multivariable logistic regression model adjusted for age, sex, insurance type, history of diabetes and history of hypertension; #, IPTW-weighted univariable logistic model. OR, odds ratio; IPTW, inverse probability of treatment weighting; CI, confidence interval; ICU, intensive care unit.
There existed no effect modification by age, sex, history of autoimmune diseases, cancer and HIV, and history of autoimmune disease (Table 3). The results of the sensitivity analysis remained consistent with the main analysis (Tables S2-S5). When redefining the study population to include all confirmed patients with COVID-19 (IPTW OR =1.33; 95% CI: 1.07–1.65), changing the exposure ascertainment window to 90 days (IPTW OR =1.43; 95% CI: 1.15–1.77), and applying other statistical methods [IPTW with trimming (IPTW OR =1.32; 95% CI: 1.06–1.63), outcome adjustment model (OR =1.29; 95% CI: 1.05–1.58), SMR weighting (OR =1.30; 95% CI: 1.03–1.64), and PS matching (OR =1.32; 95% CI: 1.07–1.64)], immunosuppressants use was associated with an increased odd of the primary endpoint consistent with the main analysis (Table S2).
Table 3
| Characteristic | N | Cumulative incidence (%) | IPTW adjusted OR | |||
|---|---|---|---|---|---|---|
| Non-user | User | OR (95% CI) | P value | |||
| Total | 4,349 | 311 (10.4) | 185 (13.6) | 1.32 (1.06–1.63) | 0.01 | |
| Age group (years) | P for interaction =0.24 | |||||
| <65 | 2,944 | 132 (6.4) | 66 (7.5) | 1.15 (0.83–1.59) | 0.39 | |
| ≥65 | 1,405 | 179 (19.4) | 119 (24.7) | 1.49 (1.21–2.00) | <0.01 | |
| Sex | P for interaction =0.23 | |||||
| Male | 1,609 | 145 (12.9) | 93 (19.3) | 1.48 (1.08–2.01) | 0.01 | |
| Female | 2,740 | 166 (8.9) | 92 (10.5) | 1.14 (0.85–1.52) | 0.39 | |
| History of autoimmune disease, cancer and HIV | P for interaction =0.95 | |||||
| No | 3,883 | 284 (10.3) | 143 (12.8) | 1.25 (0.99–1.58) | 0.05 | |
| Yes | 466 | 27 (12.1) | 42 (17.4) | 1.28 (0.72–2.26) | 0.39 | |
| History of autoimmune disease | P for interaction =0.99 | |||||
| No | 4,097 | 303 (10.4) | 161 (13.7) | 1.28 (1.02–1.59) | 0.03 | |
| Yes | 252 | 8 (10.7) | 75 (13.6) | 1.28 (0.52–3.13) | 0.58 | |
OR, odds ratio; IPTW, inverse probability of treatment weighting; CI, confidence interval.
When assessing the component outcomes individually, immunosuppressants users were at higher odds of all-cause mortality while redefining the study population to include all confirmed patients with COVID-19 (IPTW OR =1.67; 95% CI: 1.23–2.26), changing the exposure ascertainment window to 90 days (IPTW OR =1.85; 95% CI: 1.36–2.52), and applying other statistical methods [IPTW with trimming (IPTW OR =1.65; 95% CI: 1.21–2.26), outcome adjustment model (OR =1.59; 95% CI: 1.18–2.13), SMR weighting (OR =1.51; 95% CI: 1.05–2.16), PS matching (OR =1.57; 95% CI: 1.14–2.17)] (Tables S3-S5).
Among 1,356 immunosuppressants users in the main analysis, 1,340 (98.8%) were corticosteroid users. They were at increased odds of the primary endpoint (IPTW OR =1.33; 95% CI: 1.07–1.64) (Table S2). When assessing the component outcomes individually, corticosteroids users were only at higher odds of all-cause mortality—IPTW OR =1.67; 95% CI: 1.22–2.76 (Tables S3-S5), which is consistent with the analysis for immunosuppressants use.
Discussion
This study reports on a nationwide cohort of South Korean COVID-19 patients. This dataset was completely enumerated and statistically controlled for confounding using PSs. The results suggest that patients administered immunosuppressants were at increased odds of all-cause mortality, mechanical ventilation and ICU admission.
Our results support the guideline from the Center for Disease Control and Prevention in the United States, classifying patients with prior use of corticosteroids and other immunosuppressive medication as a high risk group of patients for severe COVID-19 infection (12). In support of this assertion are papers by Brenner et al. (13), Michelena et al. (14), Di Giorgio et al. (15), Marlais et al. (16) and Montero-Escribano et al. (17).
Notably, corticosteroids are known to reduce mortality, mechanical ventilation and duration of hospital stay relative to standard-of-care in patients with severe and critical COVID-19, as noted by the living systematic review and meta-analysis by Siemieniuk et al. (18) and WHO clinical guideline (7). Andersen et al. (19) reported contrary results to our paper, in that adverse outcomes were not associated with immunosuppressants use before COVID-19. However, their paper reported on a sicker patient population who might have received benefit from corticosteroids, whereas our analysis reports on a nationwide cohort of all patients admitted for COVID-19 to South Korean hospitals, which include patients with non-severe COVID-19 infection. In South Korea in the time interval assessed, typically all COVID-19 patients were admitted to hospitals, even if they were asymptomatic or had mild symptoms, in an attempt to limit spread of the COVID-19 infection. As a result, our study provides a more complete picture with all spectrum of COVID-19 patients, from asymptomatic to critical COVID-19. As corticosteroids use is beneficial in patients with severe to critical stages in COVID-19 infection and could possibly be harmful in patients with non-severe COVID-19 infection, as seen from RECOVERY trial (8), it comes as no surprise that our study results show increased odds of primary endpoint or mortality in immunosuppressants or corticosteroid users given that most COVID-19 infections are non-severe (20).
While not directly assessed, our study results support the receipt of booster COVID-19 vaccinations for immunocompromised people taking immunosuppressant medications. These findings also urge caution around blanket-continuation of immunosuppressants for COVID-19 patients who were on immunosuppressants prior to COVID-19 diagnosis; patients might need to be assessed on a per-case basis, weighing the risk of adverse COVID-19 outcomes with the benefits of continuing immunosuppressants. Consideration may need to be given to lowering the degree of immunosuppression if a patient was on immunosuppressants and has a non-severe COVID-19 infection, although establishing a dose response to immunosuppressants was not possible with the current cohort dataset.
There are several notable strengths of this study. As South Korea used a strict nationwide patient management system for COVID-19, the use of a population-based cohort mitigated any potential sampling bias issue; 94.3% of all confirmed COVID-19 patients ≥40 years of age (n=4,610) were hospitalized (n=4,349) regardless of presence of symptom or severity of disease in our study. There could be very little chance of outcome misclassification since outcomes such as all-cause death and outcomes defined from procedures codes (i.e., ICU admissions and mechanical ventilation use) are unlikely to be misclassified in the main analysis restricted to hospitalized COVID-19 patients, given the cross-referencing of these records with national death records and reimbursement review processes, respectively. Consistent results across rigorous statistical methods, including IPTW, PS matching, SMR and thorough sensitivity analyses, suggest our results are robust. Additionally, including chronic co-medications as confounders could have mitigated healthy user bias.
This study was also not without limitations. We do not have data regarding the severity of COVID-19 at the time of COVID-19 diagnosis, which is a limitation of using claims data. In addition, there may still exist residual confounding by confounders that are typically not captured in a claims database (e.g., body mass index, baseline blood pressure, laboratory test values). Also, our result is limited by the observational nature of our study design. At last but not least, the involvement of interstitial lung disease (ILD), an important aggravating factor of COVID-19 patients, could not be adjusted in this study because the frequency of ILD in this dataset was rare.
In conclusion, our study of a large nationwide cohort of hospitalized COVID-19 patients in South Korea finds that use of immunosuppressants or corticosteroids increases the odds of all-cause mortality, mechanical ventilation and ICU admissions. These findings lend credence to the latest guidelines from the CDC that people on immunosuppressants are at high risk of severe COVID-19 and could benefit from booster COVID-19 vaccinations.
Acknowledgments
The authors thank healthcare professionals dedicated to treating COVID-19 patients in South Korea, the Ministry of Health and Welfare, the Health Insurance Review & Assessment Service (HIRA) and Ye-Jin Sohn (HIRA) for sharing invaluable national health insurance claims data.
Funding: YGC’s work was supported, in part, by 2020R1G1A1A01006229 awarded by the National Research Foundation of Korea.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-21-3465/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-21-3465/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-21-3465/coif). CBS serves as an unpaid Editor-In-Chief of Annals of Palliative Medicine. YGC’s work was supported, in part, by 2020R1G1A1A01006229 awarded by the National Research Foundation of Korea. The other authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lai CC, Shih TP, Ko WC, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents 2020;55:105924. [Crossref] [PubMed]
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol 2020;5:536-44. [Crossref] [PubMed]
- Chow R, Simone CB 2nd, Lock M. Hydroxychloroquine for the treatment of COVID-19: the importance of scrutiny of positive trials. Ann Palliat Med 2020;9:3716-20. [Crossref] [PubMed]
- Baden LR, Rubin EJ. Covid-19 - The Search for Effective Therapy. N Engl J Med 2020;382:1851-2. [Crossref] [PubMed]
- Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033-4. [Crossref] [PubMed]
- Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 2020;395:473-5. [Crossref] [PubMed]
- Update to living WHO guideline on drugs for covid-19. BMJ 2020;371:m4475. [PubMed]
- RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med 2021;384:693-704. [Crossref] [PubMed]
- Republic of Korea Ministry of Health and Welfare (MoHW). COVID-19 Response: Korean government’s response system 2021. Available online: http://ncov.mohw.go.kr/en/baroView.do?brdId=11&brdGubun=111
- Freemantle N, Marston L, Walters K, et al. Making inferences on treatment effects from real world data: propensity scores, confounding by indication, and other perils for the unwary in observational research. BMJ 2013;347:f6409. [Crossref] [PubMed]
- Imbens GW, Rubin DB. Causal Inference for Statistics, Social, and Biomedical Sciences. Cambridge: Cambridge University Press, 2015.
- Centers for Disease Control and Prevention. Underlying medical conditions associated with high risk for severe COVID-19: information for healthcare providers 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html
- Brenner EJ, Ungaro RC, Gearry RB, et al. Corticosteroids, But Not TNF Antagonists, Are Associated With Adverse COVID-19 Outcomes in Patients With Inflammatory Bowel Diseases: Results From an International Registry. Gastroenterology 2020;159:481-91.e3. [Crossref] [PubMed]
- Michelena X, Borrell H, López-Corbeto M, et al. Incidence of COVID-19 in a cohort of adult and paediatric patients with rheumatic diseases treated with targeted biologic and synthetic disease-modifying anti-rheumatic drugs. Semin Arthritis Rheum 2020;50:564-70. [Crossref] [PubMed]
- Di Giorgio A, Nicastro E, Speziani C, et al. Health status of patients with autoimmune liver disease during SARS-CoV-2 outbreak in northern Italy. J Hepatol 2020;73:702-5. [Crossref] [PubMed]
- Marlais M, Wlodkowski T, Vivarelli M, et al. The severity of COVID-19 in children on immunosuppressive medication. Lancet Child Adolesc Health 2020;4:e17-8. [Crossref] [PubMed]
- Montero-Escribano P, Matías-Guiu J, Gómez-Iglesias P, et al. Anti-CD20 and COVID-19 in multiple sclerosis and related disorders: A case series of 60 patients from Madrid, Spain. Mult Scler Relat Disord 2020;42:102185. [Crossref] [PubMed]
- Siemieniuk RA, Bartoszko JJ, Ge L, et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ 2020;370:m2980. [Crossref] [PubMed]
- Andersen KM, Mehta HB, Palamuttam N, et al. Association Between Chronic Use of Immunosuppresive Drugs and Clinical Outcomes From Coronavirus Disease 2019 (COVID-19) Hospitalization: A Retrospective Cohort Study in a Large US Health System. Clin Infect Dis 2021;73:e4124-30. [Crossref] [PubMed]
- Kim AY, Gandhi RT. COVID-19: Management in hospitalized adults. UpToDate 2021. Available online: https://www.uptodate.com/contents/covid-19-management-in-hospitalized-adults/


