
Effects of the hemolytic index on the test results of a dry chemistry analyzer and a verification of the hemolytic interference threshold
Introduction
Hemolysis is one of the most common endogenous interference factors and the leading cause of sample rejections in laboratories (1,2). Sample hemolysis can lead to inaccurate test results and also affect physicians’ diagnoses (3). Additionally, hemolysis can increase the pain of patients, as it can require the re-drawing of blood, prolong the reporting period, and the re-testing of samples can also cause losses in human, physical, and financial resources (4).
The way of receiving, testing and rejecting hemolytic samples is a difficult problem in laboratory work (5). For emergency test samples, not only is the test time more urgent, but there is also a need for “concession testing” (6). The concept of “concession testing” was first proposed in the 2019 Consensus of Chinese Experts on the Construction and Standardization of Emergency Medical Laboratory Capacity, and is defined as a test that is performed by analysts as required by a clinician in a situation in which the samples are difficult to obtain or the patients are seriously ill, even if the samples would be unqualified under routine conditions; however, the results of any concession tests are for the clinician’s reference only (7).
Traditionally, interference is judged by a visual assessment of the sample’s appearance to determine the status of the sample (e.g., the presence and severity of the hemolysis). However, this method is both time consuming and subjective (8,9). Precise chemical analyzers can now automatically test serum hemolysis and express the results of the tests using the hemolytic index (10,11). The hemolytic threshold refers to the value of hemoglobin concentration at which hemolysis causes a change in the test results beyond the allowable deviation and varies depending on the test items, test systems, and test methods. When the hemolytic index of a sample is higher than the hemolytic threshold of the test item, the sample should be handled in a reasonable manner (5,12). Thus, the laboratories should verify the hemolytic threshold of test items, explore the validity and accuracy of the hemolysis results, and issue reasonable hemolysis test reports (7,13). In this study, the hemolytic thresholds of dry chemistry-based biochemical indicators were verified using the hemolytic index test function of the Ortho Vitros5600 (Ortho Clinical Diagnostics, New York, USA), and the results revealed that hemolysis interfered with 17 indicators. We present the following article in accordance with the MDAR reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-292/rc).
Methods
Instruments and reagents
A total of 26 dry chemistry-based biochemical indicators and their hemolytic indexes were tested using the Ortho Vitros5600 (Ortho Clinical Diagnostics) and its supporting kits.
Sample collection and preparation
The remaining serum samples of patients who underwent physical examinations at our laboratory were selected for testing. The samples had results within the normal range, and had not been used in other studies. A total of 3 remaining tubes of serum were selected from the same patient who had undergone a physical examination. Of these, 1 was included in the control group, and 2 were included in the test group. In this study, 1 tube of serum from the test group was aspirated 20 times with a 5-mL syringe needle to produce severe hemolysis and then centrifuged at 3,000 g for 10 min to extract hemolytic serum for future use. The hemolytic serum obtained by the manual intervention was proportionally mixed with another tube of serum from the test group to prepare different degrees of hemolytic samples. A total of 4 groups of hemolytic samples were prepared; the hemolytic index of the first group was 100–130; the hemolytic index of the second group was 200–230; the hemolytic index of the third group was 300–330; the hemolytic index of the fourth group was 400–430. In total, 20 normal serum samples and 20 hemolytic serum samples were selected from the control group and test group, respectively. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Clinical Research Ethics Committee of Dongfang Hospital, Beijing University of Chinese Medicine (No. JDF-IRB-2019033501). In this study, patients’ residual samples were tested in the laboratory, with patient information hidden, and informed consent was exempted with the approval of the ethics committee.
Testing
Once the hemolytic samples of the test group had been prepared, the normal serum samples from the control group and the samples with different degrees of hemolysis from the 4 test groups were simultaneously tested for the hemolytic indexes, and for the following 26 dry chemistry-based biochemical indicators using dry chemical reagents of the same batch: glucose (GLU), uric acid, creatinine, blood urea nitrogen, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALKP), gamma glutamyl transpeptidase (γ-GGT), cholinesterase (CHE), total protein (TP), albumin (ALB), total bilirubin (TBIL), creatine kinase (CK), creatine kinase MB (CK-MB), lactate dehydrogenase (LDH), potassium (K+), sodium, chloride, calcium, magnesium (Mg), phosphorus (PHOS), iron (Fe), carbon dioxide (CO2), cholesterol (CHO), triglyceride, and direct high-density lipoprotein (HDL) CHO.
Statistical analysis
All the data were statistically analyzed using GraphPad Prism 8.2.1 software. The measurement data are expressed as the mean ± standard deviation, while the enumeration data are expressed as the (%). A one-way analysis of variance with a Dunnett’s multiple comparisons test was used for the inter-group comparison. P value <0.05 indicated a significant difference. For the items with statistical differences, the percentage deviation of the test results between the hemolytic samples and control samples was calculated. The following formula was used to calculate the percentage deviation = (the test result of hemolytic samples – the test result of control samples) ÷ the test result of control samples ×100%. The deviation was compared with the specification [1/3 of the total error allowable (TEa)] as per the quality assessment criterion of the National Center for Clinical Laboratories. The hemolytic index with a test result deviation >1/3 of TEa was used as the warning threshold for hemolytic interference.
Results
Of the 26 test items, hemolysis interfered with 17 items. Among them, hemolysis positively interfered with the test results of PHOS, CK, γ-GGT, Mg, Fe, TP, K+, TBIL, LDH, ALB and AST. As described in the manufacturer’s statement, when γ-GGT activity was 73 U/L and the hemoglobin interference concentration reached 600 mg/dL, the enzyme activity of γ-GGT decreased by 13 U/L, resulting in a negative deviation. However, the test data showed that hemolysis positively interfered with the result of γ-GGT, but negatively interfered with the test results of CHE, direct HDL, GLU, CO2, ALKP, and ALT. TP, CO2, TBIL, LDH, CHE, ALB, and AST in the 4 test groups with different degrees of hemolysis were statistically different to those in the control group; CK, Mg, GLU, ALT, and ALKP in the 200-, 300-, and 400-haemolytic index groups differed significantly to those in the control group; PHOS, γ-GGT and direct HDL in the 300- and 400-haemolytic index groups differed significantly to those in the control group; Fe in the 400-haemolytic index group differed significantly to that in the control group (see Table 1). Among the 17 test items interfered with by hemolysis (with the exception of Mg and direct HDL for which the test results in the 100- and the 200-haemolytic index groups did not exceed the allowable 1/3 TEa compared to the control group), the deviation of the other test results in the test groups with different degrees of hemolysis exceeded the allowable 1/3 TEa compared to the control group (see Table 2, and Figure 1).
Table 1
| Test indicators | Normal group | Hemolytic group | ||||
|---|---|---|---|---|---|---|
| Hemolytic index <15 (example number =20) | Hemolytic index =100 (example number =20) | Hemolytic index =200 (example number =20) | Hemolytic index =300 (example number =20) | Hemolytic index =400 (example number =20) | ||
| PHOS (mmol/L) | ||||||
| Value | 1.39±0.06 | 1.53±0.19 | 1.64±0.32 | 1.76±0.51 | 1.85±0.63 | |
| P value | – | 0.6197 | 0.1517 | 0.0149 | 0.0015 | |
| CK (U/L) | ||||||
| Value | 86.50±16.71 | 99.13±17.35 | 107.90±16.92 | 116.00±15.35 | 124.00±16.60 | |
| P value | – | 0.0603 | 0.0004 | <0.0001 | <0.0001 | |
| γ-GGT (U/L) | ||||||
| Value | 23.41±6.13 | 24.62±6.16 | 26.67±6.12 | 29.01±6.2 | 30.18±6.56 | |
| P value | – | 0.9295 | 0.293 | 0.0203 | 0.0034 | |
| Mg (mmol/L) | ||||||
| Value | 0.89±0.04 | 0.914±0.04 | 0.93±0.05 | 0.96±0.05 | 0.98±0.05 | |
| P value | – | 0.2626 | 0.0262 | <0.0001 | <0.0001 | |
| Fe (μmol/L) | ||||||
| Value | 22.33±7.41 | 25.17±8.43 | 26.66±9.05 | 28.36±9.72 | 30.04±10.49 | |
| P value | – | 0.6709 | 0.3096 | 0.0864 | 0.0172 | |
| dHDL (mmol/L) | ||||||
| Value | 1.73±0.20 | 1.69±0.22 | 1.58±0.20 | 1.51±0.17 | 1.46±0.18 | |
| P value | – | 0.8586 | 0.0583 | 0.0019 | <0.0001 | |
| GLU (mmol/L) | ||||||
| Value | 5.60±0.31 | 5.30±0.39 | 4.88±0.55 | 4.54±0.70 | 4.26±0.76 | |
| P value | – | 0.2732 | 0.0005 | <0.0001 | <0.0001 | |
| TP (g/L) | ||||||
| Value | 86.13±2.60 | 90.73±2.62 | 93.38±2.38 | 96.39±1.97 | 98.99±1.79 | |
| P value | – | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| K+ (mmol/L) | ||||||
| Value | 4.60±0.16 | 5.78±0.31 | 6.88±0.69 | 8.02±1.00 | 9.09±1.47 | |
| P value | – | 0.0002 | <0.0001 | <0.0001 | <0.0001 | |
| CO2 (mmol/L) | ||||||
| Value | 23.25±1.71 | 21.80±1.62 | 21.4±1.39 | 20.46±1.67 | 19.79±1.76 | |
| P value | – | 0.0217 | 0.0021 | <0.0001 | <0.0001 | |
| TBIL (μmol/L) | ||||||
| Value | 15.35±2.11 | 20.84±2.35 | 27.95±2.46 | 34.91±2.12 | 41.24±2.91 | |
| P value | – | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| ALKP (U/L) | ||||||
| Value | 81.72±7.42 | 73.47±10.82 | 71.72±12.72 | 72.59±12.78 | 70.78±14.38 | |
| P value | – | 0.0979 | 0.0313 | 0.0565 | 0.0158 | |
| LDH (U/L) | ||||||
| Value | 386.10±34.86 | 742.80±65.53 | 1,044.00±95.97 | 1,298.00±110.60 | 2,040.00±200.90 | |
| P value | – | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| CHE (U/L) | ||||||
| Value | 8,832.0±633.9 | 7,940.0±653.0 | 7,346.0±453.2 | 7,017.0±448.3 | 6,831.0±484.2 | |
| P value | – | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| ALB (g/L) | ||||||
| Value | 51.97±1.47 | 54.35±1.70 | 57.10±4.70 | 58.44±1.51 | 59.18±2.38 | |
| P value | – | 0.0197 | <0.0001 | <0.0001 | <0.0001 | |
| AST (U/L) | ||||||
| Value | 26.39±3.40 | 38.54±5.09 | 50.90±6.49 | 63.33±7.98 | 75.20±8.70 | |
| P value | – | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| ALT (U/L) | ||||||
| Value | 29.72±8.21 | 26.22±9.09 | 18.78±9.00 | 14.06±8.88 | 11.17±7.76 | |
| P value | – | 0.5082 | 0.0005 | <0.0001 | <0.0001 | |
ALB, albumin; ALKP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate amino-transferase; CHE, cholinesterase; CK, creatine kinase; dHDL, direct high-density lipoprotein cholesterol; CO2, carbon dioxide; Fe, iron; GLU, glucose; K+, potassium; LDH, lactate dehydrogenase; Mg, magnesium; PHOS, phosphorus; TBIL, total bilirubin; TP, total protein; γ-GGT, gamma glutamyl transpeptidase.
Table 2
| Judgment standard and group | Project mean deviation (%) in the hemolytic group | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PHOS | LDH | CK | γ-GGT | Mg | Fe | CHE | dHDL | GLU | TP | ALB | K+ | CO2 | TBIL | AST | ALKP | ALT | |
| Total TEa | 10 | 11 | 15 | 11 | 15 | 15 | 20 | 30 | 7 | 5 | 6 | 6 | 15 | 15 | 15 | 16 | 16 |
| 1/3 TEa | 3.33 | 3.67 | 5.00 | 3.67 | 5.00 | 5.00 | 6.67 | 10.00 | 2.33 | 1.67 | 2.00 | 2.00 | 5.00 | 5.00 | 5.00 | 5.33 | 5.33 |
| H-100 | 10.23 | 92.91 | 19.30 | 5.63 | 2.84 | 13.53 | −10.09 | −2.69 | −5.29 | 5.40 | 4.63 | 25.87 | −6.16 | 36.63 | 46.38 | −10.42 | −13.01 |
| H-200 | 17.89 | 171.94 | 29.80 | 14.87 | 4.40 | 20.35 | −16.73 | −8.51 | −12.77 | 8.47 | 9.99 | 49.72 | −7.75 | 83.64 | 93.76 | −12.78 | −39.54 |
| H-300 | 26.21 | 238.63 | 39.57 | 25.69 | 8.49 | 28.39 | −20.47 | −12.59 | −18.76 | 11.97 | 12.50 | 74.67 | −11.82 | 130.36 | 141.66 | −11.74 | −55.59 |
| H-400 | 32.88 | 432.48 | 48.95 | 30.99 | 10.41 | 36.09 | −22.62 | −15.85 | −23.84 | 15.00 | 13.96 | 98.01 | −14.71 | 171.69 | 186.88 | −14.14 | −64.92 |
ALB, albumin; ALKP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate amino-transferase; CHE, cholinesterase; CK, creatine kinase; CO2, carbon dioxide; dHDL, direct high-density lipoprotein cholesterol; Fe, iron; GLU, glucose; H, hemolytic index; K+, potassium; LDH, lactate dehydrogenase; Mg, magnesium; PHOS, phosphorus; TBIL, total bilirubin; TP, total protein; γ-GGT, gamma glutamyl transpeptidase; TEa, total error allowable.
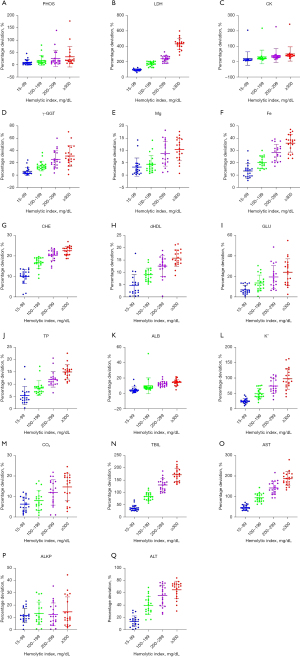
Discussion
When hemolysis occurs in a sample, red blood cells destroy the release of the cellular contents, and further interfere with the test results via optical interference, chemical reactions with the reagent components, and other mechanisms, resulting in falsely increased or decreased test results, which can have adverse effects on clinical diagnosis and treatment (9,14). At present, there are no particularly good measures to eliminate the interference of hemolysis index. Most clinical laboratories reject or re-collect specimens to reduce the interference of hemolysis on test results. The identification of hemolytic interference using the hemolytic index, instead of a visual assessment, enables the quality of the hemolytic samples to be assessed in an accurate and objective manner, improves the accuracy of the test results, and solves the interference caused by the clinical hemolytic samples (15). At present, there are a lot of clinical laboratory biochemical immunity analyzer have hemolysis index detection function and according to the different equipment brand or methodology setting up different hemolysis interference threshold, so the same test project in different test system on the interference of hemolysis threshold may not be the same. For example, Abbott Architect Biochemical system (C4000/8000/16000), Beckman AU System, Hitachi Chemistry, Roche biochemical detection system (Cobas C 701/C 702, etc.), Mindray biochemical test system (BS-2000m/BS-2800m), the above instruments of hemolysis index test method are colorimetric method. Each test system verifies its own hemolysis index threshold using artificially produced specimens of different levels of hemolysis.
Based on domestic and foreign research (16,17), and given the realities of our domestic laboratories, the hemolysis ratio is usually calculated by a hemolytic index >50 (equivalent to serum/plasma hemoglobin ≥0.5 g/L) using an instrumental method, and the hemolysis ratio for outpatients is generally 0.2–0.4% as calculated by the hemolytic index, and 0.4–0.8% in inpatients and even higher in emergency patients. Reasonable hemolysis thresholds should be established so that when the hemolytic index of any test item is higher than the corresponding threshold, the necessity of the re-sampling of the hemolytic samples can be evaluated in combination with practical clinical needs. This measure would reduce the rejection rate of hemolytic samples, improve the satisfaction of physicians and patients, and simplify the workflow in laboratories. When the hemolytic index is tested in laboratories, it is necessary to verify the hemolytic interference threshold and the trend of changes in the test results caused by hemolysis interference.
In this study, the hemolytic thresholds of the items tested by the Ortho Vitros5600 (Ortho Clinical Diagnostics) were verified using the hemolytic index test function of the dry chemistry analyzer via a comparison of the test results between the control group and test groups with different degrees of hemolysis to obtain more accurate data under hemolytic interference, and examine the trend of changes in the test items under hemolytic interference through the verification of hemolytic thresholds, and test the hemolytic samples in a reasonable manner. The hemolytic thresholds of the 26 biochemical indicators examined in this study revealed that hemolysis interfered with 17 of them; thus, the test results of 9 indicators were not affected by hemolysis. It has been reported that hemolysis index has an effect on the results of insulin (INS), neuron specific enolase (NSE), folic acid (FOL) and ferritin (FER), adrenocorticotropic hormone (ACTH), procalcitonin (PCT), parathyroid hormone (PTH), pro-gastrin-releasing peptide (ProGRP). Negative interference to test results of ACTH, ProGRP, PCT, PTH, INS; Positive interference to test results of NSE and FOL. However, it should be noted that compared to immune indicators, biochemical indicators are more likely to be affected by hemolysis due to their reaction characteristics and the composition of the interfering substances.
We analyzed the trend of hemolytic interference, but found that the trend for the hemolysis effect differed across different test items. In addition to the false increase of K+ or LDH results, hemolysis also positively interfered with PHOS, CK, γ-GGT, Mg, Fe, TP, TBIL, ALB, and AST, but negatively interfered with other items. As some items may have different effect trends to those stated in manufacturers’ statements, it is important to verify and establish reasonable hemolytic thresholds in individual laboratories. Additionally, we need to examine whether these deviations can be clinically accepted if 1/3 of the TEa is set as the reference standard. However, in this study, the interference caused by hemolysis could not be eliminated, the specific deviation of the test items caused by hemolysis could not be calculated, and only the trend of interference with test items caused by hemolysis could be determined.
The hemolytic thresholds of test items should be used in a reasonable manner in laboratories. The information related to the hemolytic index, hemolytic interference threshold, and clinical diagnosis should be reviewed before the test reports for any hemolytic samples are issued (5,18). If the hemolytic index is lower than the hemolytic threshold of the test item, the laboratory should report the test results in a reasonable manner instead of rejecting the sample. If the hemolytic index is higher than the hemolytic threshold of the test item, the laboratory may recommend clinical re-sampling for testing, or report the test results exceeding the hemolytic threshold. Explanatory notes on the sample hemolysis or test results under hemolytic interference are required if there is a clinical need for concession testing. For example, the information about which items are interfered with by hemolysis, resulting in an increase or decrease in test results, should be noted in the laboratory information system (Beijing Zhifang Technology Development Co., Ltd., China), so that clinicians can obtain more detailed results information of hemolytic samples. We used the function of hemolysis index to automatically add remarks in the test report and send out the hemolysis assessment test report, which reduced the rejection rate of clinical hemolysis specimens by 0.2%. More than 90% of the medical staff said that the hemolysis index can improve the quality of hemolysis specimen testing, shorten the specimen turnaround time (TAT), save costs and reduce medical disputes. The biggest disadvantage is that there is no clear detection method to determine the specific value of hemolysis interference, so it is impossible to quantify the interference degree.
Under the principle of priority diagnosis and treatment, all laboratories should constantly seek to improve the accuracy of laboratory test results, reduce the rejection rates of samples, and issue reasonable assessments and test reports of hemolytic samples to improve the test quality of hemolytic samples, shorten the TAT time, save costs, and reduce medical disputes.
Acknowledgments
The authors would like to sincerely thank colleagues in their laboratory Chengwei Ding for guiding the collection of the data and the statistical analysis, and Ge Zheng for guiding the writing of the manuscript and data checking.
Funding: This work was supported by the Scientific Research Fund of Beijing University of Chinese Medicine (No. 2019-JYB-JS-152).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-292/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-292/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-292/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Clinical Research Ethics Committee of Dongfang Hospital, Beijing University of Chinese Medicine (No. JDF-IRB-2019033501). In this study, patients’ residual samples were tested in the laboratory, with patient information hidden, and informed consent was exempted with the approval of the ethics committee.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lippi G, Blanckaert N, Bonini P, et al. Haemolysis: an overview of the leading cause of unsuitable specimens in clinical laboratories. Clin Chem Lab Med 2008;46:764-72. [Crossref] [PubMed]
- Lippi G, Salvagno GL, Montagnana M, et al. Influence of hemolysis on routine clinical chemistry testing. Clin Chem Lab Med 2006;44:311-6. [Crossref] [PubMed]
- Robbiano C, Birindelli S, Dolci A, et al. Impact of managing affected results in haemolysed samples of an infant-maternity hospital using an unconventional approach. Clin Biochem 2021;95:49-53. [Crossref] [PubMed]
- Wan Azman WN, Omar J, Koon TS, et al. Hemolyzed Specimens: Major Challenge for Identifying and Rejecting Specimens in Clinical Laboratories. Oman Med J 2019;34:94-8. [Crossref] [PubMed]
- Ishiguro A, Nishioka M, Morishige A, et al. What is the best wavelength for the measurement of hemolysis index? Clin Chim Acta 2020;510:15-20. [Crossref] [PubMed]
- Phelan MP, Hustey FM, Good DM, et al. Seeing Red: Blood Sample Hemolysis Is Associated with Prolonged Emergency Department Throughput. J Appl Lab Med 2020;5:732-7. [Crossref] [PubMed]
- Domino L, Christensen PA. Serum index rules prevent risk of analysing uncentrifuged tubes on automated biochemistry analysers. Scand J Clin Lab Invest 2021;81:511-6. [Crossref] [PubMed]
- Barbhuiya MA, Pederson EC, Straub ML, et al. Automated Measurement of Plasma Cell-Free Hemoglobin Using the Hemolysis Index Check Function. J Appl Lab Med 2020;5:281-9. [Crossref] [PubMed]
- Calvaresi EC, La'ulu SL, Snow TM, et al. Plasma hemoglobin: A method comparison of six assays for hemoglobin and hemolysis index measurement. Int J Lab Hematol 2021;43:1145-53. [Crossref] [PubMed]
- Ercan M, Akbulut ED, Bayraktar N, et al. Effects of specimen haemolysis on complete blood count results by Abbott Alinity hq System. Biochem Med (Zagreb) 2021;31:030706. [Crossref] [PubMed]
- Monneret D, Mestari F, Atlan G, et al. Hemolysis indexes for biochemical tests and immunoassays on Roche analyzers: determination of allowable interference limits according to different calculation methods. Scand J Clin Lab Invest 2015;75:162-9. [Crossref] [PubMed]
- Van Elslande J, Hijjit S, De Vusser K, et al. Delayed diagnosis and treatment of extreme hypertriglyceridemia due to rejection of a lipemic sample. Biochem Med (Zagreb) 2021;31:021002. [Crossref] [PubMed]
- Kalaria T, Gill H, Harris S, et al. The effect of haemolysis on the direct and indirect ion selective electrode measurement of sodium. Ann Clin Biochem 2021;58:190-5. [Crossref] [PubMed]
- Cao Y, Branzell I, Vink M. Determination of clinically acceptable cut-offs for hemolysis index: An application of bootstrap method using real-world data. Clin Biochem 2021;94:74-9. [Crossref] [PubMed]
- Ilardo C, Lancien A, Barthes J. Study of haemolysis interference limit on serum potassium assay on Roche® Cobas 8000 and evaluation of corrected potassium. Scand J Clin Lab Invest 2021;81:82-4. [Crossref] [PubMed]
- Clifford-Mobley O, Sheerin S. Verifying the haemolysis index limit for non-reporting potassium. Ann Clin Biochem 2021;58:385-7. [Crossref] [PubMed]
- Ni J, Zhu W, Wang Y, et al. A Reference chart for clinical biochemical tests of hemolyzed serum samples. J Clin Lab Anal 2021;35:e23561. [Crossref] [PubMed]
- Mondejar R, Mayor Reyes M, Melguizo Madrid E, et al. Utility of icteric index in clinical laboratories: more than a preanalytical indicator. Biochem Med (Zagreb) 2021;31:020703. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


