
Study on the clinical efficacy of bone-filled mesh vertebroplasty combined with posterior screw and rod internal fixation in the treatment of thoracolumbar metastases: a retrospective cohort study
Introduction
Spine metastases often occur in patients with malignant tumors. The metastatic sites are the thoracic spine (about 70%), lumbar spine (about 20%), and cervical spine (about 10%). As spinal metastases are dominated by osteolytic destruction, they lead to a decrease in the strength of the vertebral body. In the early stage, strong back pain commonly occurs, and in the later stage, spinal instability and paralysis caused by spinal cord compression occur, which severely reduces the patient’s end-stage quality of life (1). How to select a surgical procedure that can restore the stability of the spine and improve the quality of life of patients with spinal metastases, while reducing surgical trauma and taking into account safety and efficacy, is an urgent problem to be solved. For this reason, from January 2018 to April 2020, we used bone-filled mesh vertebroplasty combined with posterior decompression and nail/rod internal fixation to treat thoracolumbar metastases, and achieved good results. We present the following article in accordance with the STROBE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-25/rc).
Methods
Participants
This is a retrospective cohort study. We retrospectively analyzed the clinical data of 68 patients with thoracolumbar vertebral metastases admitted to Sun Yat-sen Memorial Hospital of Sun Yat-sen University from January 2018 to April 2020. The inclusion criteria were as follows: (I) non-primary tumors of the spine, with clear pathological diagnosis; (II) patients with persistent thoracic and dorsal pain >1 month, which was not relieved by standard conservative treatment; the main lesion vertebral body was located in the thoracic spine, lumbar spine, or thoracolumbar region. Imaging confirmed the existence of bone destruction (2), spinal cord and nerve compression, according to the magnetic resonance imaging (MRI) T2-weighted image of the epidural tumor on the spinal cord compression degree (3) [epidural spinal cord compression (ESCC)] above grade 2; (III) Tomita score 4–7 points (4), the expected survival time was more than 1 year; (IV) spinal instability neoplastic score (SINS) >7 points (5). (V) Frankel score B–E. The exclusion criteria were as follows: (I) patients with poor cardiopulmonary, liver, and kidney function, who were unable to tolerate surgery, or had poor mental status and were unable to cooperate with treatment; (II) difficult to correct blood coagulation mechanism; (III) systemic infection such as sepsis and skin infection at the surgical site; (IV) severe anemia and hypoproteinemia.
In the study group, 37 cases were treated with bone-filled mesh vertebroplasty combined with posterior screw and rod internal fixation. According to Frankel grade classification, 12 cases were graded as E, 10 cases were D grade, 8 cases were C grade, and 7 cases were B grade. In the control group, 31 cases were treated with conventional vertebroplasty combined with posterior screw and rod internal fixation. The Frankel classification system graded 11 cases as grade E, 9 cases in grade D, 7 cases in grade C, and 5 cases in grade B. General participant information is shown in Table 1. Both X-ray and computed tomography (CT) were used to determine the changes in the height of the affected vertebral body before the operation, and the height of the anterior edge of the vertebral body and the height of the middle edge of the vertebral body were recorded. The relative height of the diseased vertebra = the height of the diseased vertebra/the height of the normal vertebra × 100%. The general information of the 2 groups of participants is shown in Table 1.
Table 1
| Project | Observation group | Control group |
|---|---|---|
| Number of cases | 37 | 31 |
| Male to female ratio | 11:26 | 10:21 |
| Average age (years) | 60.9±8.7 | 58.6±7.9 |
| Source of primary lesion: breast cancer (case) | 19 | 15 |
| Source of primary tumor: lung cancer (cases) | 8 | 7 |
| Source of primary focus: liver cancer (case) | 4 | 5 |
| Source of primary tumor: prostate cancer (case) | 6 | 4 |
| Transfer vertebral body (section) | 42 | 35 |
| VAS score (points) | 5.62±0.73 | 5.74±0.69 |
VAS, visual analogue scale.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of Sun Yat-sen Memorial Hospital of Sun Yat-sen University (No. SYSEC-KY-KS-2022-076). Individual consent for this retrospective analysis was waived.
Surgical methods and postoperative treatment
All participants underwent general anesthesia and were placed in the prone position. The diseased vertebrae was located by C-arm machine fluoroscopy, and we used posterior median approach for surgery. First, the pedicle screw was inserted in the upper and lower segments of the diseased vertebra, the nerve roots on both sides of the diseased vertebra were decompressed, and the puncture needle for pedicle puncture was then inserted. In the observation group, we inserted a bone-filled mesh bag (Shandong Guanlong Co., Shandong, China) through a puncture needle. Then, we prepared bone cement, of which we injected about 1 mL under X-ray monitoring when the bone cement had been prepared for 4 minutes. When the bone cement had just entered the mesh bag, we proceeded to inject 0.5 mL of bone cement. The injection was then paused for 1 minute, after which we continued the injection until the bone cement was evenly dispersed, then removed the working channel. In the control group, we used an ordinary vertebroplasty kit (Shandong Guanlong Co.) for pedicle puncture, injected bone cement, and immediately stopped the injection after leakage. A titanium rod was installed, horizontally connected, and fixed. The drainage tube was inserted according to routine protocol and the incision was closed layer by layer. After the operation, specimens were routinely subjected to pathological biopsy to further confirm the diagnosis. The storage time of the drainage tube was determined according to the drainage volume, with a maximum duration of no more than 1 week. Cases were treated symptomatically with anti-inflammatory, dehydration, nerve nutrition, fluid rehydration, and so on, after surgery, re-examination of X-ray, CT, and MRI after operation. After the operation, the patient was instructed to walk gradually with the protection of a brace according to the nerve function of the lower limbs. According to the condition of the primary tumor, cases were treated with radiotherapy, chemotherapy and other medical oncology adjuvant treatments. Re-examination and follow-up were performed at 3 months, 6 months, and 1 year after surgery.
Observation index
The visual analogue scale (VAS) (https://eportfolios.macaulay.cuny.edu/reisf16/files/2016/09/pain-scale-visual.pdf) was used to evaluate the improvement of patients’ subjective pain symptoms (6). The Oswestry disability index (ODI) (https://www.britishspineregistry.com/app/download/27959933/Oswestry+Disability+Index.pdf) was used to evaluate the improvement of patients’ motor function status (6). The Karnofsky performance status (KPS) scale (http://www.npcrc.org/files/news/karnofsky_performance_scale.pdf) was used to assess the improvement of patients’ quality of life (7). The patient’s VAS score, ODI score, and KPS score before surgery, 3 days after surgery, 3 months after surgery, 6 months, and 1 year after surgery were recorded. We used X-ray and CT to record the height of the anterior edge of the vertebral body and the height of the middle edge of the vertebral body in the follow-up 1 week after the operation, 6 months after the operation, and 1 year after the operation. The Frankel grade was assessed 1 year after surgery.
Statistical methods
The software SPSS 20.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis (8). Normally distributed data were expressed as mean ± standard deviation () (9). The comparison between each interval before and after the operation used repeated measures analysis of variance, and least significant difference (LSD) test was used for pairwise comparison. Rank sum test was used for comparison before and after surgery, and the inspection level α=0.05. A 2 independent sample t-test was used for comparison between groups, and a paired-material t-test was used for intra-group comparison. Count data were expressed as a percentage (%), the chi-squared (χ²) test was used for comparison between groups. If P<0.05, the difference was considered statistically significant.
Results
There were no statistically significant differences in gender, age, and VAS score between the 2 groups. The total number of fixed segments in the observation group was 168, and the number of fixed segments in the control group was 156. There was no statistically significant difference in operation time and intraoperative bleeding between the 2 groups. All cases were followed up for 6–12 months. The pain VAS scores of the 2 groups at 1 week, 3 months, 6 months, and 1 year after surgery were statistically significant compared with those before surgery. The ODI scores of the 2 groups were statistically significant at 1 week, 3 months after surgery, 6 months after surgery, and 1 year after surgery. The postoperative KPS score gradually increased with time, and it was statistically significant compared with the preoperative comparison. There was no statistical significance in the comparison between the 2 groups at the same time period. The details are described in Tables 2,3. The anterior and middle heights of the diseased vertebrae were compared before the operation, 1 week, 3 months, 6 months, and 1 year after the operation. The comparison between the 2 groups at the same time period after surgery was statistically significant (Table 4). At 1 year after the operation, the control group had 14 cases of Frankel classification E, 16 cases of D, 4 cases of C, and 3 cases of class B; the observation group had 12 cases of Frankel classification E, 14 cases of D, 3 cases of C, and 2 B grade cases.
Table 2
| Group | Number of cases | VAS score (points) | ODI score (points) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Preoperative | One week after surgery | Three months after surgery | Six months after surgery | One year after surgery | Preoperative | One week after surgery | Three months after surgery | Six months after surgery | One year after surgery | |||
| Observation group | 37 | 7.2±1.5 | 2.6±1.2 | 1.5±0.7 | 1.3±0.6 | 1.1±0.8 | 75.5±9.3 | 26.8±3.9 | 24.1±5.6 | 23.6±5.9 | 21.7±4.8 | |
| Control group | 31 | 6.9±1.3 | 2.5±1.4 | 1.3±0.6 | 1.4±0.5 | 1.3±0.7 | 76.3±6.8 | 27.4±5.1 | 23.8±4.5 | 24.7±5.2 | 22.3±4.5 | |
| t | 0.034 | 0.998 | 0.508 | 0.188 | 0.905 | 0.681 | 0.547 | 0.882 | 0.807 | 0.753 | ||
| P value | 0.973 | 0.452 | 0.899 | 0.319 | 0.932 | 0.258 | 0.953 | 0.533 | 0.600 | 0.687 | ||
VAS, visual analogue scale; ODI, Oswestry disability index.
Table 3
| Group | Number of cases | KPS score (points) | ||||
|---|---|---|---|---|---|---|
| Preoperative | One week after surgery | Three months after surgery | Six months after surgery | One year after surgery | ||
| Observation group | 37 | 63.3±7.5 | 82.6±6.9 | 88.1±5.2 | 89.6±6.4 | 89.8±5.6 |
| Control group | 31 | 62.6±8.2 | 81.3±5.7 | 86.6±4.8 | 87.5±5.6 | 86.9±6.3 |
| t | 0.840 | 0.141 | 0.577 | 0.031 | 0.312 | |
| P value | 0.788 | 0.939 | 0.333 | 0.515 | 0.522 | |
KPS, Karnofsky performance status.
Table 4
| Group | Number of cases | The relative height of the anterior edge of the injured vertebra | Central vertebral relative height | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Preoperative | One week after surgery | Three months after surgery | Six months after surgery | One year after surgery | Preoperative | One week after surgery | Three months after surgery | Six months after surgery | One year after surgery | |||
| Observation group | 37 | 58.3±8.9 | 86.5±7.3 | 85.9±6.7 | 83.4±8.1 | 81.6±7.5 | 57.7±7.6 | 84.9±6.4 | 83.6±5.7 | 81.2±7.1 | 80.9±5.8 | |
| Control group | 31 | 56.9±6.4 | 72.3±6.5 | 71.4±7.2 | 70.9±5.7 | 68.1±6.1 | 56.3±5.9 | 75.2±6.8 | 73.7±6.5 | 72.4±5.9 | 70.6±7.0 | |
| t | 2.602 | 9.218 | 7.213 | 10.213 | 10.267 | 0.839 | 12.144 | 15.029 | 11.813 | 14.329 | ||
| P value | 0.011 | 0.000 | 0.000 | 0.000 | 0.000 | 0.404 | 0.000 | 0.000 | 0.000 | 0.000 | ||
In the observation group, there were 4 cases of 6 vertebral bodies that had bone cement leakage during the operation, the leakage rate was 14.29%; the control group had 8 cases of 11 vertebral bodies that had bone cement leakage during the operation, and the leakage rate was 31.43%. The difference was statistically significant. There were no complications such as wound infection and internal fixation breakage in either group.
Typical case presentation
A 54-year-old female patient with breast cancer and multiple thoracic vertebral body metastases underwent T3–T11 pedicle screw fixation + T5, T8 bilateral bone-filled mesh pocket vertebroplasty (Figure 1).
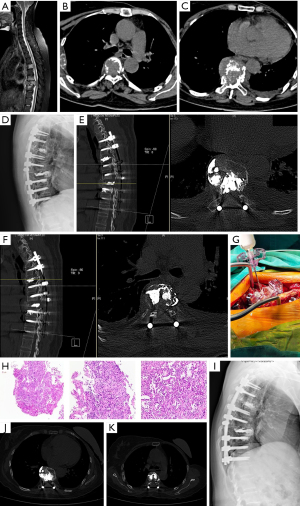
Discussion
Spinal metastasis is a very common disease; bones are one of the most vulnerable tissues, and the spine is the most frequently affected part of the bones (10). The treatment of spinal metastases has become a hot topic to reduce pain-related neurological symptoms and surgery-related complications (11), along with individualized precision radiotherapy (12). Multi-treatment methods have been applied to treat spinal metastasis, like operation, radiotherapy, bisphosphonates and denosumab, embolization, and radiofrequency thermal ablation, electrochemotherapy and high-intensity focused ultrasound (13). With the continuous advancement of various treatment methods, the survival time of patients with spinal metastases has been prolonged. The purpose of surgical intervention should be as follows: (I) provide immediate stability and allow weight bearing; (II) fixation of the fracture; (III) fix the patient for their whole life; and (IV) under reasonable circumstances, stabilize all lesions of the affected bone (14).
Surgical methods commonly used to treat spinal metastases include percutaneous vertebroplasty, radical resection and reconstruction, lamina decompression, and pedicle screw fixation. Percutaneous vertebroplasty involves the use of polymethylmethacrylate (PMMA) bone cement to fill the fractured vertebral body. It can improve the compressive strength of the injured vertebral body, avoid collapse and deformity, and relieve pain. The pain relief rate is 75–94% (15). The infusion of bone cement causes necrosis of nerve endings or tumor tissue in the vertebrae by blocking the supporting veins of the vertebrae (16,17). When bone cement releases heat, the local temperature reaches 70 ℃, which can directly kill tumor cells, and the cytotoxicity of its monomer also has a killing effect on tumors, bone cement packaging can further reduce the spread of vertebral tumor cells (18), therefore, the incidence of related complications is very low (19). However, vertebroplasty alone cannot achieve the purpose of decompression, and the incidence of bone cement leakage is 30–87.5% (20).
For patients with spinal metastases accompanied by spinal cord compression and pathological compression fractures of the vertebral body, radical resection and reconstruction with anterior approach or combined anterior and posterior approach is selected. Although it can effectively control tumor dissemination and reduce the rate of local recurrence, intraoperative reconstruction of spine stability is more complicated, and there are more bleeding incidents, more complications, and a long learning curve. Further, extensive resection may stimulate tumor growth by activating blood vessel production (21).
Patients with spinal metastases usually have comorbidities such as malnutrition, weakened immune system, severe pain, and limited overall survival rate, whereby extensive surgical procedures or extended hospital stays are unacceptable (22). For patients with pre-existing primary tumors, physical and mental pressure is heavy, and most patients are unable or unwilling to undergo extensive vertebral body resection and reconstruction surgery. Based on this situation, we chose to perform partial resection of the vertebral tumor and release of the compressed nerve roots from the posterior median approach incision, while using a polyethylene terephthalate (PET) mesh bag. The PET structure can encase most of the bone cement, thereby greatly reducing the risk of bone cement leakage outside the vertebral body (1,23).
In this group of cases, the total laminectomy of the diseased vertebrae + bilateral nerve root decompression was performed routinely during the operation to achieve the goal of adequate decompression (24). After nerve root decompression, the Frankel classification of most patients had improved compared with preoperatively, especially for those who had just developed spinal cord compression symptoms. These findings were supportive of intraoperative laminectomy + nerve root decompression having a good effect. However, in this group of cases, bone cement leakage occurred in 3 vertebral body operations, and the leakage rate was 14.29%, which was lower than 31.43% in the control group, indicating that the use of bone-filled mesh bags is safer (25). The relative height of the anterior and middle part of the diseased vertebrae and the sagittal position of the observation group recovered significantly 1 week after the operation, and there was no significant loss of vertebral body height during follow-up from 6 months to 1 year after surgery, which was statistically significant compared with the control group. This shows that pedicle screw and rod internal fixation combined with bone-filled mesh vertebroplasty can effectively reconstruct the stability of the spine.
The postoperative VAS score and ODI scores of the observation group were significantly lower than those before the operation, and the KPS score gradually increased over time, indicating that the patient’s postoperative pain was sufficiently relieved and the quality of life improved; however, the comparison between the 2 groups was not statistically significant, indicating that both surgical procedures can achieve good results. However, the sample size of this group of cases was small, and most of them were breast cancer metastases for which the expected survival period is long. Coupled with the short follow-up time, this study had certain limitations. Multi-center comparison studies are needed in future to validate our findings (expected sample size to reach more than 300 cases). More random primary tumors and longer follow-up (follow-up for more than 5 years) are also needed to verify the long-term efficacy.
The application of bone filled mesh bag is especially suitable for patients with bone metastases with vertebral posterior wall defect. The bone filled mesh bag is composed of polyethylene terephthalate interlaced with each other to form a mesh bag structure. It is biocompatible and non-stretchable. A deflated bone-filled mesh bag is placed directly into the vertebra, which is filled with bone cement and expands to its desired shape. The bone cement from the mesh can bond with the bone trabeculae in the vertebral body, so that the bone cement and the vertebral body can be perfectly combined. The bone cement from the mesh can bond with the bone trabeculae in the vertebral body, so that the bone cement and the vertebral body can be perfectly combined. The position of bone cement in the vertebral body can be observed by intraoperative CT or X-ray. It can reduce the probability of bone cement leakage and is of high safety. The main disadvantage is expensive.
Conclusions
The surgical treatment technique of treating thoracolumbar metastases with bone-filled mesh vertebroplasty combined with posterior screw and rod internal fixation is mature, and it can not only reduce the impact of the operation on the patient, but also reduce the risk of bone cement leakage. At the same time, it can achieve the purpose of relieving pain, restoring nerve function, and rebuilding spinal stability. For patients with metastatic tumors of the thoracic and lumbar spine accompanied by spinal cord compression, especially metastatic tumors with damaged posterior wall of the vertebral body, this surgical method provides an effective and relatively safe treatment plan.
Acknowledgments
Funding: The study was supported by Scientific Research Funding of Tianjin Medical University Chu Hsien-I Memorial Hospital (No. 2018ZDKF10), Guangzhou Science and Technology Project (No. 202102020096) and Regenerative Medicine and Health Laboratory of Guangzhou (1102101201).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-25/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-25/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-25/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of Sun Yat-sen Memorial Hospital of Sun Yat-sen University (No. SYSEC-KY-KS-2022-076). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Reyad RM, Ghobrial HZ, Hakim SM, et al. Thick cement usage in percutaneous vertebroplasty for malignant vertebral fractures at high risk for cement leakage. Diagn Interv Imaging 2017;98:721-8. [Crossref] [PubMed]
- Wagner A, Haag E, Joerger AK, et al. Cement-Augmented Carbon Fiber-Reinforced Pedicle Screw Instrumentation for Spinal Metastases: Safety and Efficacy. World Neurosurg 2021;154:e536-46. [Crossref] [PubMed]
- Bilsky MH, Laufer I, Fourney DR, et al. Reliability analysis of the epidural spinal cord compression scale. J Neurosurg Spine 2010;13:324-8. [Crossref] [PubMed]
- Tokuhashi Y, Matsuzaki H, Oda H, et al. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976) 2005;30:2186-91. [Crossref] [PubMed]
- Fisher CG, DiPaola CP, Ryken TC, et al. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine (Phila Pa 1976) 2010;35:E1221-9. [Crossref] [PubMed]
- Gündoğdu Z, Öterkuş M, Karatepe Ü. Evaluation of the Effect of Radiofrequency Denervation on Quality of Life of Patients with Facet Joint Syndrome by Oswestry Disability Index Score and Visual Analogue Scale Score. Prague Med Rep 2021;122:278-84. [Crossref] [PubMed]
- Goto Y, Hino A, Hashimoto N. A comparative analysis of the modified Rankin Scale, Karnofsky Performance Status and Kurtzke expanded disability status scale in the perioperative management of patients with brainstem cavernous malformations. Clin Neurol Neurosurg 2021;207:106785. [Crossref] [PubMed]
- Wang T, Li B, Wang Z, et al. Sorafenib promotes sensory conduction function recovery via miR-142-3p/AC9/cAMP axis post dorsal column injury. Neuropharmacology 2019;148:347-57. [Crossref] [PubMed]
- Li Y, Zou J, Li B, et al. Anticancer effects of melatonin via regulating lncRNA JPX-Wnt/β-catenin signalling pathway in human osteosarcoma cells. J Cell Mol Med 2021;25:9543-56. [Crossref] [PubMed]
- Witham TF, Khavkin YA, Gallia GL, et al. Surgery insight: current management of epidural spinal cord compression from metastatic spine disease. Nat Clin Pract Neurol 2006;2:87-94; quiz 116. [Crossref] [PubMed]
- Colangeli S, Capanna R, Bandiera S, et al. Is minimally-invasive spinal surgery a reliable treatment option in symptomatic spinal metastasis? Eur Rev Med Pharmacol Sci 2020;24:6526-32. [PubMed]
- Gottumukkala S, Srivastava U, Brocklehurst S, et al. Fundamentals of Radiation Oncology for Treatment of Vertebral Metastases. Radiographics 2021;41:2136-56. [Crossref] [PubMed]
- Tsukamoto S, Kido A, Tanaka Y, et al. Current Overview of Treatment for Metastatic Bone Disease. Curr Oncol 2021;28:3347-72. [Crossref] [PubMed]
- Challapalli A, Aziz S, Khoo V, et al. Spine and Non-spine Bone Metastases - Current Controversies and Future Direction. Clin Oncol (R Coll Radiol) 2020;32:728-44. [Crossref] [PubMed]
- Health Quality Ontario. Vertebral Augmentation Involving Vertebroplasty or Kyphoplasty for Cancer-Related Vertebral Compression Fractures: A Systematic Review. Ont Health Technol Assess Ser 2016;16:1-202.
- Wang TY, Yang ZZ, Chen JH, et al. Clinical study of percutaneous vertebroplasty combined with (125)I seeds implantation in the treatment of patients with thoracic metastatic tumor complicated with posterior vertebral defect. Zhonghua Zhong Liu Za Zhi 2020;42:1056-62. [PubMed]
- Liu Y, Wang Y, Zhao L, et al. Effectiveness and safety of percutaneous vertebroplasty in the treatment of spinal metastatic tumor. Pak J Med Sci 2017;33:675-9. [Crossref] [PubMed]
- Kim HJ, Suh BG, Lee DB, et al. The influence of pain sensitivity on the symptom severity in patients with lumbar spinal stenosis. Pain Physician 2013;16:135-44. [PubMed]
- Long Y, Yi W, Yang D. Advances in Vertebral Augmentation Systems for Osteoporotic Vertebral Compression Fractures. Pain Res Manag 2020;2020:3947368. [Crossref] [PubMed]
- Zhang J, Wang Y, Han K, et al. Percutaneous vertebroplasty combined with zoledronic acid for the treatment of painful osteolytic spinal metastases in patients with breast cancer. J Vasc Interv Radiol 2013;24:1861-7. [Crossref] [PubMed]
- Schaefer C, Schroeder M, Fuhrhop I, et al. Primary tumor dependent inhibition of tumor growth, angiogenesis, and perfusion of secondary breast cancer in bone. J Orthop Res 2011;29:1251-8. [Crossref] [PubMed]
- Harel R, Angelov L. Spine metastases: current treatments and future directions. Eur J Cancer 2010;46:2696-707. [Crossref] [PubMed]
- Seo SS, Lee DH, Kim HJ, et al. Percutaneous vertebroplasty at C7 for the treatment of painful metastases -A case report-. Korean J Anesthesiol 2013;64:276-9. [Crossref] [PubMed]
- Hansen-Algenstaedt N, Kwan MK, Algenstaedt P, et al. Comparison Between Minimally Invasive Surgery and Conventional Open Surgery for Patients With Spinal Metastasis: A Prospective Propensity Score-Matched Study. Spine (Phila Pa 1976) 2017;42:789-97. [Crossref] [PubMed]
- Nieuwenhuijse MJ, Van Erkel AR, Dijkstra PD. Cement leakage in percutaneous vertebroplasty for osteoporotic vertebral compression fractures: identification of risk factors. Spine J 2011;11:839-48. [Crossref] [PubMed]
(English Language Editor: J. Jones)


