A case report of bone metastases from appendiceal adenocarcinoma and a review of literature
Introduction
The incidence of appendiceal adenocarcinoma (AA) is extremely low, accounting for less than 0.5% of gastrointestinal neoplasms (1). Given the rarity of this disease, there is a very small sample size, resulting in a lack of guiding literature regarding the efficacy of treatments available to health care professionals.
AAs are differentiated into three different subtypes: mucinous, colonic type and signet cell type (2). Although appendiceal carcinoids were considered to be the more populous subtype of appendiceal cancer, there is emerging evidence suggesting the prevalence of histologically diagnosed AA is increasing (2).
Appendiceal cancers commonly exhibit rupture of the appendix, and peritoneal disease [e.g., peritoneal carcinomatosis, pseudomyxoma peritonei (PMP)] (1). Outside the peritoneum, the most common site of AA metastases reported is the lung (3), although these are scarce as well. Management of colorectal cancer (CRC) and by extension, appendiceal cancer, has evolved, with 10- and 15-year survivals being reported in literature. Given these developments, more cases of hematogenic or lymphatic spread to distant organs may surface in the future.
This report follows the rare case of a 50-year-old male with node-negative mucinous AA who developed and was treated with palliative radiotherapy for symptomatic pelvic bone metastases. To our understanding, such a case of bone metastases with mucinous AA has only been reported twice in literature. Huck and Shen (3), as well as Rockwood and Brecher (4) reported cases of lumbar metastases from a confirmed primary mucinous AA (3) and an AA arising from a mucocele (4).
Case presentation
A 50-year-old male with mucinous AA was referred to the Rapid Response Radiotherapy Program at Sunnybrook Health Sciences Centre in July 2015 for pain in the hips and left leg.
He initially presented with hematuria in 2013 and was found to have had an urachal tumor. Pathology upon resection unexpectedly revealed a moderately differentiated T4b mucinous AA. The patient underwent a right hemicolectomy in July 2013. Final pathology showed no evidence of residual malignancy with all 29 lymph nodes (LN) testing negative. With a high risk stage II diagnosis, the patient underwent 8 cycles of adjuvant oral capecitabine.
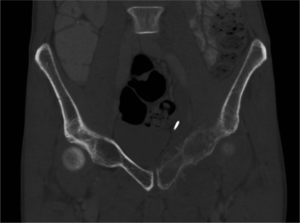
In May 2015, computed tomography (CT) imaging of the abdomen and pelvis showed lytic involvement of the left pubic bone (Figure 1) with thickening of the adjacent soft tissue (Figure 2). It also demonstrated metastatic lymphatic involvement. A biopsy of this region confirmed recurrence of AA. The patient underwent 30 Gy of external beam radiation treatment (EBRT) in 10 fractions to the pelvis for palliation. Radiation elicited a good response in pain, eliminating the need for routine analgesics 4 weeks post-treatment.

Discussion
Presently, AA is treated with a variety of treatment modalities, including appendectomies (5), hemicolectomies (5), as well as neoadjuvant and adjuvant chemotherapies (e.g., 5-FU based FOLFOX and FOLFIRI regimens) (6). As use of cytoreductive surgery (CRS) and heated intraperitoneal chemotherapy (HIPEC) have been increasingly advocated for in the treatment of gastrointestinal cancers, CRS/HIPEC has also been considered and employed in the treatment of AA with peritoneal disease (7).
There are no specific guidelines regarding systemic chemotherapies for AAs, and thus therapies have largely been extrapolated from the established treatment of CRC (7). Despite there being little high-level evidence available on the efficacy of systemic chemotherapies in this population, 5-FU-based regimens have been recommended and are largely drafted in the neoadjuvant or adjuvant treatment of this cancer.
In the absence of randomized control trials, there are several small retrospective studies on this topic. Some studies demonstrate that patients with poor prognostic factors still stand to benefit from chemotherapy. In their retrospective review of patients with metastatic, poorly differentiated/signet cell AA, Lieu et al. reported a 44% radiographic response as well as an improved median overall survival (OS) of 1.7 years and a progression free survival (PFS) of 6.9 months (8). A study conducted at M.D. Anderson with 52 AA patients who were deemed suboptimal candidates for CRS reported that 56% received a clinical benefit (complete response, partial response or stable disease) and a median PFS and OS of 7.6 months and 56 months respectively. Most patients in the study received 5-FU or capecitabine (9).
These relatively small, retrospective studies have been limited to regimens based on namely one agent—5-FU, and lack a diverse range of cytotoxic or biologic therapies in modern chemotherapy. Tejani et al. conducted a retrospective study analyzing patients with metastatic AA in the CRC database from 2005–2012. Out of this population, 45% received systemic therapy, with the most commonly employed regimens being FOLFOX (with or without bevacizumab), FOLFORI and 5-FU. Of evaluable patients, there was a 39% response rate and 36% rate of stability in disease. Mucinous type AA was associated with higher PFS and OS. Patients who underwent non-debulking surgical interventions had worse PFS and OS. Results showed that AA patients undergoing systemic chemotherapy achieved similar response rates of PFS and OS as CRC patients (6). For this reason, the National Comprehensive Cancer Network (NCCN) recommends that health care providers consider prognostic factors in individual cases and refer to the appropriate treatment guidelines in colon cancer (7).
While appendectomies, hemicolectomies, and CRS/HIPEC have all been shown to increase survival in patients, there is conflict surrounding the minimal disease characteristics necessitating utilization of one treatment over the other. Walters et al. suggested that while hemicolectomies may increase survival in those with diseases of later stages, appendectomies may otherwise be sufficient (5). However, debulking surgeries such as CRS are recommended for the treatment in AA patients with peritoneal disease (e.g., PMP). Studies suggest that mucinous AA, when compared to other GI cancers, is more responsive to CRS (10). Chua et al. reported 10- and 15-year survival rates of 63% and 59% respectively for patients with mucinous AA and PMP who underwent CRS/HIPEC in their retrospective study. While CRS is typically used as first line treatment in patients with resectable peritoneal disease, Chua et al. also advocated for the use of CRS despite of high volume disease. Completeness of CRS was also associated with higher survival (10). CRS is now the most commonly used surgical intervention in patients with appendiceal cancer (7).
Recommendations have been made of ensuring adequate LN sampling (>12) upon resection (11) and surveillance through frequent thoracic imaging. Depending on prognostic factors in individual cases, optimal surgical resection and systemic chemotherapies should be considered.
The efficacy and applicability of other treatment modalities such as vascular endothelial growth factor (VEGF) inhibitors, and other molecularly targeted therapies (e.g., bevacizumab, cetuximab) have not yet been as extensively studied in this patient population.
The impact of other treatment modalities on this primary cancer has not been well reported on. There has been one retrospective study evaluating the response of localized disease to post-operative EBRT, an average of 45.5 Gy in 1.8 fractions, in conjunction with 5-FU based chemotherapy. The results from the study suggest the use of radiation for local control, especially in those with tumor-associated bowel perforation or other prognostic factors indicative of a higher risk of recurrence. Fifty percent (5/10) patients failed after receiving solely a surgical intervention; whereas 20% (1/5) failed after receiving post-operative radiation treatment (12).
Conclusions
Due to the lack of high-level evidence surrounding the treatment of a diverse AA population, the onus is on health care providers to deliver what they deem to be appropriate therapies for their patients. Effective selection of patients for certain treatment modalities requires the consideration of prognostic factors such as LN status, histologic grade, subtype and metastases.
It is also important to consider the associated morbidities of the chemotherapies (e.g., 5-FU, oxaliplatin, etc.) and surgical interventions (e.g., CRS, hemicolectomies, etc.) available in the treatment of AA. Although the benefits of these therapies have been substantiated in studies, effective selection of patients is necessary in order to maximize gains in terms of OS and minimize losses in terms of morbidities and unfavorable side-effects. Future studies regarding the efficacy of modern chemotherapeutic agents and other treatment modalities, such as EBRT, in the management of AA are warranted.
Acknowledgements
We thank the generous support of Bratty Family Fund, Michael and Karyn Goldstein Cancer Research Fund, Joey and Mary Furfari Cancer Research Fund, Pulenzas Cancer Research Fund, Joseph and Silvana Melara Cancer Research Fund, and Ofelia Cancer Research Fund.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
- Nitecki SS, Wolff BG, Schlinkert R, et al. The natural history of surgically treated primary adenocarcinoma of the appendix. Ann Surg 1994;219:51-7. [Crossref] [PubMed]
- Turaga KK, Pappas SG, Gamblin T. Importance of histologic subtype in the staging of appendiceal tumors. Ann Surg Oncol 2012;19:1379-85. [Crossref] [PubMed]
- Huck MB, Shen P. Bone metastases in appendiceal adenocarcinoma. Am Surg 2014;80:e274-5. [PubMed]
- Rockwood E, Brecher FR. Primary adenocarcinoma of the appendix with metastasis to the fifth lumbar vertebra: report of a case and review of the English-language literature. J Am Osteopath Assoc 1971;70:681-5. [PubMed]
- Walters KC, Paton BL, Schmelzer TS, et al. Treatment of appendiceal adenocarcinoma in the United States: penetration and outcomes of current guidelines. Am Surg 2008;74:1066-8. [PubMed]
- Tejani MA, ter Veer A, Milne D, et al. Systemic therapy for advanced appendiceal adenocarcinoma: an analysis from the NCCN Oncology Outcomes Database for colorectal cancer. J Natl Compr Canc Netw 2014;12:1123-30. [PubMed]
- Benson AB, Venook AP, Bekaii-Saab T, et al. Colon cancer, version 2.2015: featured updates to the NCCN Guidelines. National Comprehensive Cancer Network. Avaialable online: http://www.tri-kobe.org/nccn/guideline/colorectal/english/colon.pdf
- Lieu CH, Lambert LA, Wolff RA, et al. Systemic chemotherapy and surgical cytoreduction for poorly differentiated and signet ring cell adenocarcinomas of the appendix. Ann Oncol 2012;23:652-8. [Crossref] [PubMed]
- Shapiro JF, Chase JL, Wolff RA, et al. Modern systemic chemotherapy in surgically unresectable neoplasms of appendiceal origin: a single-institution experience. Cancer 2010;116:316-22. [Crossref] [PubMed]
- Chua TC, Moran BJ, Sugarbaker PH, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol 2012;30:2449-56. [Crossref] [PubMed]
- Bunni J, Arnold D, Shelley-Fraser G, et al. Multidisciplinary perspective on the management of appendiceal adenocarcinoma: case review of 10 patients from a university hospital and current considerations. Clin Colorectal Cancer 2015;14:58-62. [Crossref] [PubMed]
- Proulx GM, Willett CG, Daley W, et al. Appendiceal carcinoma: patterns of failure following surgery and implications for adjuvant therapy. J Surg Oncol 1997;66:51-3. [Crossref] [PubMed]


