Extremely rare presentation of soft tissue metastasis from carcinoma breast as a massive swelling of upper extremity
Introduction
Metastasis to soft tissue from a primary malignancy is very rare (1) with few case series (2-9) and isolated case reports to verify it. It can easily be misdiagnosed as a soft tissue sarcoma (STS) or an inflammatory lesion (5,6). It is invariable to distinguish between a metastatic soft tissue tumor from other diagnostic pathologies as the management pattern and prognosis in all these entities are different and any overlap may cause unnecessary morbidity (4). Histopathological and immunohistochemical (IHC) analysis plays the major role in differentiating and determining the primary lesion. Metastasis to soft tissues is seen primarily in solid tumors like lungs, stomach, colon, esophagus, uterus and pancreas (5) and haematological malignancies like lymphomas (6) which have shown more propensities for soft tissue spread as compared to carcinoma breast (6). Till date, breast carcinomas metastasizing to soft tissue, whether skeletal muscle or subcutaneous tissue has been rarely reported (4,9,10,11) while only 6 previous cases of upper limb involvement have been described by Plaza et al. (4), Surov et al. (9) and Konatam et al. (10) thus establishing it as a rare entity and its early diagnosis and treatment are important for a better prognosis.
Case presentation
An 80-year-old female presented with history of progressive painful swelling of right upper arm and shoulder of 2 months duration. A thorough history revealed her to be an old case of carcinoma right breast treated with upfront modified radical mastectomy (MRM), adjuvant chemotherapy and loco-regional radiotherapy (LRRT) followed by hormonal therapy 15 years ago.
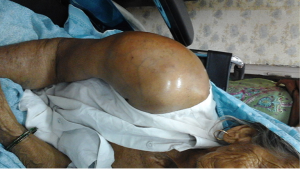
Local examination showed a massive spherical swelling involving flexor and extensor aspect of right upper arm and shoulder measuring 21 cm × 17 cm with a shiny and stretched overlying skin. The swelling was firm to hard in consistency, tender, immobile, fixed to underlying structures and was without any pitting edema (Figure 1). Right chest wall showed an old healed surgery scar while left breast was unremarkable. There was no evidence of any local or generalized lymphadenopathy.
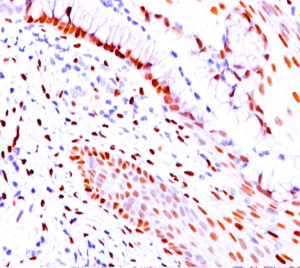
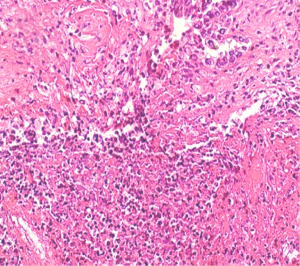
Trucut biopsy from the swelling showed metastatic deposits from a poorly differentiated adenocarcinoma (Figure 2). Metastatic workup with whole body positron emission (PET) scan did not show any primary lesion. Further IHC examination of the soft tissue mass revealed tumor cells staining positive for estrogen (Figure 3) and progesterone receptors (ER/PR) while negative for HER 2-neu, desmin, epithelial membrane antigen (EMA), CK 7 and thyroid transcription factor 1 (TTF-1), suggestive of primary from breast carcinoma.
Patient was advised disarticulation of right shoulder joint by onco-surgeons in view of a dysfunctional limb but she refused for the same. She was eventually planned for local palliative radiotherapy (RT) to alleviate pain and swelling but after 3 days of starting RT she lost to treatment and did not follow up.
Discussion
Soft tissue metastasis from any primary site has been rarely reported in world literature till date (2-9) while breast as the primary malignancy has been reported by still fewer studies (4,9,10,11). With regard to soft tissue metastasis, some studies have reported a frequency of 0.8% based on autopsies (12) while few reported an incidence of 0.2% based on clinical studies (13). This rarity can be due to the fact that soft tissues produce anti-carcinogenic factors like lactic acid, beta adrenergic receptors or protease inhibitors which serve as a deterrent for metastatic invasion (1,9,14). Soft tissue metastasis can be in subcutaneous tissue or in muscular tissue (5).
Regarding the primary neoplasm, only Plaza et al. (4) reported 13 cases/11% cases of breast carcinomas as the primary, 16% cases of skin carcinoma, 11% cases of lung carcinoma, 10% colon and 10% kidney carcinomas in 118 cases of soft tissue metastasis, while Damron et al. (5) and Torigoe et al. (6) highlighted lung carcinoma as the primary solid malignancy and lymphoma as the primary liquid malignancy apart from stomach, colon, esophagus, uterus and pancreas having the maximum tendency to metastasize to soft tissues as compared to breast (4).
Breast carcinoma generally metastasizes to bone, lung, liver, brain and lymph nodes, whereas soft tissue is not the usual site. As for the soft tissue metastatic sites, Plaza et al. (4) and Torigoe et al. (6) reported anterior abdominal wall, Damron et al. (4) identified thigh as the predominant location, Surov et al. (9) reported extraocular muscles as a potential site followed by back, chest wall and lower extremities with upper extremities being the least common site as happened in our case. Plaza et al. (4) described 3 cases, Surov et al. (9) reported 2 while Konatam et al. (10) reported 1 case of upper extremity involvement from breast cancer. As for the size of metastatic lesion, Konatam et al. (10) described a 15 cm × 7 cm swelling while we report a 21 cm × 17 cm soft tissue metastatic swelling.
Breast carcinoma is an aggressive disease with about 10–15% patients developing distant metastasis within 3–4 years, however such manifestation after 15 years is uncommon though not unusual in the present scenario of highly advanced diagnostic modalities, definitive and adjuvant treatment which has increased the life span of a patient where the metastatic state can manifest. This also implies that the patient harbours the risk of distant metastasis during their entire lifetime which is further enhanced by the heterogenous nature of the breast disease which makes it difficult to identify the risk factors and assess the curative modality.
Distant or local spread via hematogenous or lymphogenous route augers a dismal prognosis for the patient with a larger tumor size, higher grade, high lymph nodal burden and lymphovascular invasion (LVI) being the known prognostic markers. Hormone receptors studies have shown that estrogen receptor (ER) positive breast carcinomas metastasize to skeletal system (10), while HER 2-Neu overexpression is associated with poor prognosis in axillary lymph node positive patients. DNA microarray studies have shown that breast carcinoma is both a local and a systemic disease and it possesses the capability of early metastasis to distant sites. Gene-expression signatures and gene-expression profiling are the novel techniques which are being used to identify breast cancers patients likely to develop distant metastasis, which is the main cause of mortality.
As for the initial diagnosis of any soft tissue lesion, Damron et al. (5) and Torigoe et al. (6) identified STS in 43% and 50% respectively followed by a cyst or an abscess, thus establishing STS as the primary differential diagnosis. Another important clinical entity which can resemble a soft tissue swelling of upper extremities in a previously treated breast carcinoma causing a clinical dilemma is lymphedema, or lymphangiosarcoma in a chronic lymphedema case. A lymphedema is a known side effect of breast carcinoma treated with surgery and RT which damage the rich lymphatics of the breast causing lymph to accumulate in body tissues resulting in swelling of the affected organ.
Lymphedema generally develops 1 to 5 years after therapy, appears on upper extremities on the same side of the operated breast, and is generally painless, underlying veins are not visible and there is presence of pitting edema. On the other hand a metastatic soft tissue swelling will be painful due to involvement of subcutaneous tissue and skin, can appear at any site on body, underlying veins may be visible and there is no pitting edema, as it was with our case. Most importantly, to suspect a metastatic swelling, history of any prior malignancy is important as there are no clinical or radiological similarities between them apart from histologic characteristics (5).
The main diagnostic approach for identifying soft tissue metastasis has been magnetic resonance imaging (MRI) which is now being superseded by 18-fluorodeoxyglucose whole body positron emission tomography (18-FDG WB PET CT) scan due to its higher sensitivity (15) as most metastatic lesions have higher FDG uptake as compared to normal tissues. The CT component of the scan helps localize the lesions as soft tissue metastatic lesions appear hyperdense or hypodense as compared to the surrounding soft tissues.
However, only a histopathological examination of biopsy specimens verifies the diagnosis, as the metastatic lesion and the primary will have similar histological features. Further immunohistochemistry of the tissue confirms the exact site of primary lesion. Expression of desmin, CD 34, EMA, S100 indicates a STS, while immunopositivity for CK7+ (16) and/or CK20+ (16), TTF-1 signifies a pulmonary or gastro-intestinal adenocarcinoma respectively. Our case stained positive for both ER and PR while being negative for desmin, CK7, TTF-1, EMA which proved breast origin of metastasis. The diagnosis of the exact pathology is of utmost importance as the treatments of all entities are different and any misdiagnosis can result in undue morbidity or even death.
Regarding treatment of metastatic soft tissue tumors, a multimodality approach generally is adopted depending upon the performance status of the patient, any comorbid condition, type of primary malignancy, site and size of the metastatic lesion. RT and chemotherapy have been generally considered the primary modality of therapy either in combination or separately while surgery is reserved for patients not responding to radiation or chemotherapy (4,5). Damron et al. (5) reported 25% while Torigoe et al. (6) reported 47% 1-year survival rate in the combined chemoradiation modality, thus indicating the poor prognosis of patients with soft tissue metastasis.
By reporting our case we want to suggest that newer prognostic markers should be developed to identify breast carcinoma patients who are at risk for developing distant metastases. Also a better understanding and interpretation of the molecular and biological mechanism of metastasis may help to device therapeutic strategies to counter this disease process. A high degree of clinical suspicion and immuno-histopathological confirmation is required to identify and diagnose any soft tissue swelling over body in a previously treated breast primary to prevent any inappropriate treatment causing undue morbidity or even mortality.
Acknowledgements
We thank the next of kin (son) of the patient for allowing us to publish the case report and use the images taken during her stay in our institute.
We also like to extend our gratitude to department of Surgical Oncology, department of Pathology and department of Radiology, Army Hospital Research and Referral, New Delhi, India.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent to publication was obtained from the NOK of the patient before she left treatment, which has been submitted.
References
- Seely S. Possible reasons for the high resistance of muscle to cancer. Med Hypotheses 1980;6:133-7. [Crossref] [PubMed]
- Sridhar KS, Rao RK, Kunhardt B. Skeletal muscle metastases from lung cancer. Cancer 1987;59:1530-4. [Crossref] [PubMed]
- McKeown PP, Conant P, Auerbach LE. Squamous cell carcinoma of the lung: an unusual metastasis to pectoralis muscle. Ann Thorac Surg 1996;61:1525-6. [Crossref] [PubMed]
- Plaza JA, Perez-Montiel D, Mayerson J, et al. Metastases to soft tissue: a review of 118 cases over a 30-year period. Cancer 2008;112:193-203. [Crossref] [PubMed]
- Damron TA, Heiner J. Distant soft tissue metastases: a series of 30 new patients and 91 cases from the literature. Ann Surg Oncol 2000;7:526-34. [Crossref] [PubMed]
- Torigoe T, Terakado A, Suehara Y, et al. Metastatic Soft Tissue Tumors. Journal of Cancer Therapy 2011;2:746-51. [Crossref]
- Tuoheti Y, Okada K, Osanai T, et al. Skeletal muscle metastases of carcinoma: a clinicopathological study of 12 cases. Jpn J Clin Oncol 2004;34:210-4. [Crossref] [PubMed]
- Herring CL Jr, Harrelson JM, Scully SP. Metastatic carcinoma to skeletal muscle. A report of 15 patients. Clin Orthop Relat Res 1998.272-81. [Crossref] [PubMed]
- Surov A, Hainz M, Holzhausen HJ, et al. Skeletal muscle metastases: primary tumours, prevalence, and radiological features. Eur Radiol 2010;20:649-58. [Crossref] [PubMed]
- Konatam ML, Triveni B, Zeesham S, et al. Soft tissue metastasis in carcinoma breast: a rare presentation. International Journal of Scientific and Research Publications 2015;5:1-7.
- Khanna S, Prakash S, Kumar S, et al. Soft tissue metastasis in carcinoma breast: a case report. World J Pathol 2013;2:25-28.
- Pearson CM. Incidence and type of pathologic alterations observed in muscle in a routine autopsy survey. Neurology 1959;9:757-66. [Crossref] [PubMed]
- James JJ, Evans AJ, Pinder SE, et al. Bone metastases from breast carcinoma: histopathological-radiological correlations and prognostic features. Br J Cancer 2003;89:660-5. [Crossref] [PubMed]
- Djaldetti M, Sredni B, Zigelman R, et al. Muscle cells produce a low molecular weight factor with anti-cancer activity. Clin Exp Metastasis 1996;14:189-96. [PubMed]
- Pfannenberg C, Aschoff P, Schanz S, et al. Prospective comparison of 18F-fluorodeoxyglucose positron emission tomography/computed tomography and whole-body magnetic resonance imaging in staging of advanced malignant melanoma. Eur J Cancer 2007;43:557-64. [Crossref] [PubMed]
- Tot T. Cytokeratins 20 and 7 as biomarkers: usefulness in discriminating primary from metastatic adenocarcinoma. Eur J Cancer 2002;38:758-63. [Crossref] [PubMed]