
The effects of integrated palliative care on quality of life and psychological distress in patients with advanced cancer: a systematic review and meta-analysis
Introduction
Cancer rates are increasing globally, particularly in developing countries. According to the latest World Health Organization data, the global cancer burden reached 19.3 million new cases in 2020, with one in 5 people worldwide developing cancer during their lifetime, and one in 8 men and one in 11 women dying from the disease (1). As clinical symptoms are not always obvious in the early stages, most patients with malignancy are diagnosed in the late stage (2). In advanced cancer, survival is commonly <1 year (3), and the distressing symptoms most commonly reported by people include fatigue, breathlessness, pain, and anorexia (4). These symptoms usually become gradually aggravated with the tumor progression, which may result in major psychological distress and worse health-related quality of life (QoL).
Integrated palliative care (IPC) is a multidisciplinary approach that mainly aims to evaluate and treat physical, functional, psychological, social, and spiritual symptoms to improve QoL and general psychological distress of both patients and families (5,6). Currently, IPC is regarded as the standard therapy for chronic and progressive diseases, especially advanced and terminal cancer (7). Several studies have been conducted in recent years to assess the effects of IPC on mental health and QoL among terminally ill cancer patients, but the outcomes of these studies have varied. One meta-analysis focused only on integrated outpatient palliative care for patients with advanced cancer (8). Another recent meta-analysis, published in 2021, was performed on studies of multidisciplinary palliative care in advanced disease (9).
These two meta-analyses on IPC have strictly treated randomized controlled trials (RCTs) as an inclusion criterion. Fulton et al. (8). reviewed integrated outpatient palliative care for advanced cancer patients and found that short-term QoL improved (10 studies; SMD =0.24; 95% CI: 0.13 to 0.35) and symptom burden improved (five studies; SMD =−0.25; 95% CI: −0.39 to −0.11); however, there was no short-term effect on depressive symptom severity reporting as a continuous outcome (two studies; SMD =−0.09; 95% CI: −0.32 to 0.1). Oluyase et al. (9) reviewed hospital-based specialist palliative care for advanced illness and found improvement in patients’ health-related QoL (10 studies; SMD =0.26; 95% CI: 0.15 to 0.37) and patient satisfaction with care (two studies; SMD =0.36; 95% CI: 0.14 to 0.57), as well as a significant reduction in patient symptom burden (six studies, SMD =−0.26; 95% CI: −0.41 to −0.12) and patient depression (eight studies; SMD =−0.22; 95% CI: −0.34 to −0.10).
Although a previous meta-analysis also showed that IPC could reduce psychological distress and improve QoL (9,10), few meta-analyses have taken into account that the various integration models may have different effects in patients with advanced cancer. In addition, no previous meta-analysis on IPC’s long-term efficacy has been conducted. Therefore, characteristics of the time course of the efficacy of IPC on cancer-related outcomes need to be identified. To facilitate evidence-based health care, the outcomes of multiple studies must be aggregated. Thus, the purpose of this meta-analysis was to synthesize evidence from published studies to assess the effects of IPC on psychological distress and QoL among patients with advanced cancer. We present the following article in accordance with the PRISMA reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-162/rc).
Methods
Search strategy
A systematic literature search of PubMed, PsycINFO, EMBASE, CINAHL, and Cochrane Central Register of Controlled Trials was conducted from inception to August 15, 2019, and the search was updated on September 07, 2021. The search string included a combination of synonyms for neoplasms, palliative care, psychological distress, QoL, and randomized controlled trials (Appendix 1). The reference lists of the retrieved literature were further searched to identify any relevant gray literature. Two reviewers completed the screening process independently, and disagreement were resolved by the third reviewer.
Eligibility criteria
Inclusion criteria
The current meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) checklist (11). We included randomized controlled trials reporting on the effectiveness of IPC interventions provided to adult patients (≥18 years) with terminal or advanced cancer. Interventions could be conducted in any setting, including primary care settings, hospitals and community settings. Studies were included if they assessed the outcomes of psychological distress or QoL. Specific outcomes for searching the relevant literature were not limited.
Exclusion criteria
Non-randomized comparative studies and before-and-after studies were excluded. Studies that included newly diagnosed cancer patients, usually at stages I or II, were excluded. Studies that included a substantial proportion of patients with nonmalignant cancer and other illnesses were also excluded due to disparities in trajectories of decline leading to death and in patients’ physical and mental conditions (12). Given the focus of this meta-analysis on IPC in particular, rather than on spiritual interventions or psychotherapies in general, we excluded studies that applied dignity therapy and studies that connected the patients to nature or to the sacred, as well as studies of psychosocial intervention.
Literature quality evaluation
We used the Cochrane risk of bias assessment tool (13) to assess the RCTs’ methodological quality, risk of bias in selection, performance, detection, attrition, reporting, and other factors. Two independent reviewers independently appraised risk of bias and then provided a summary assessment for each study. No study was excluded as a result of findings from the risk of bias assessment.
Statistical method
Statistical analyses were performed with STATA version 15.0 software. Standardized mean differences (SMDs) were calculated for the pooled effects. All estimations are presented with their 95% confidence intervals (95% CIs). All pooled outcome measures were determined using random-effects models. The magnitude of heterogeneity among the included studies was assessed using the chi-squared test (Chi2) and I-squared statistic (I2). For the Chi2 test, a Cochran’s Q P value of <0.10 was considered significant. An I2 value of more than 75% was considered to indicate a high degree of heterogeneity, 50–75% was moderate, and 25–50% was a low degree of heterogeneity (14). Sensitivity was examined by assessing the effect of a single study on the overall pooled estimates. Publication bias was evaluated using Egger’s test, and P>0.05 represented the absence of publication bias.
Results
Study selection
Our literature database search yielded 3,644 records, and an additional search yielded 17 more records. After removing duplicates, 2,880 records remained. Of those, 2,791 records were excluded after screening titles and abstracts. Full reports of 89 publications were acquired, and 61 publications were further excluded for various reasons (Figure 1). As a result of the eligibility check, 12 articles were finally included. For a further description of our screening process, see the PRISMA study flow diagram (Figure 1).
Study characteristics
The studies were all from 5 high-income countries (United States, Denmark, Italy, Switzerland and Belgium). Ten trials (15-24) included patients with various cancers, and two trials (25,26) only included leukemia patients. Several outcomes that had been measured most frequently (i.e., overall QoL and psychological distress) were selected for our meta-analysis. Psychological distress includes depression, anxiety, and posttraumatic stress disorder (PTSD) symptoms. All included studies involved IPC as the primary intervention. We divided interventions into three models: the inpatient consulting model; the hospital outpatient model; and the model involving multiple settings. The follow-up assessments started anywhere from the completion of the intervention to half a year later (Table 1).
Table 1
| Reference | Country | Sample type | N (each group) | Age (years, mean) | Design type | Duration | Control group type | Assess period | Outcome measure |
|---|---|---|---|---|---|---|---|---|---|
| Clark et al., 2012 | America | Advanced cancer (0–50% expected 5-year survival rate) diagnosed within 12 months | 65, 66 | 58.7, 59.9 | Hospital outpatient model: structured multidisciplinary intervention | The six 90-minute sessions | Standard oncologic care | Baseline, 4 weeks, 27 weeks | FACT-G |
| McCorkle et al., 2015 | America | Advanced cancer diagnosed within 100 days | 66, 80 | 60 | Hospital outpatient model: multidisciplinary outpatient consultation team | 10 weeks | Usual care | Baseline, 1 month, 3 months | SDS, PHQ-9, ESDS, SF-12, FACT-G, HADS, MUIS-Community Form, SEMCD-6 |
| El-Jawahri et al., 2016 | America | Haematologic malignancies undergoing autologous/allogeneic HCT | 81, 79 | 57.2, 56.9 | Inpatient consulting model: integrated early palliative care | Period of hospitalisation | Standard transplant care | Baseline, 2 weeks, 3 months | FACT-BMT and Fatigue subscale, HADS, PHQ-9, ESAS, PCL-C |
| Grudzen et al., 2016 | America | Emergency Department patients with advanced cancer | 69, 67 | 55.1, 57.8 | Inpatient consulting model: IPC | Enrolment to discharge from hospital | Usual care | Baseline, 6 weeks, 12 weeks | ECOG, FACT-G, PHQ-9 |
| Groenvold et al., 2017 | Denmark | Advanced cancer | 145, 152 | 69.5 | Model involving multiple settings: integrated early palliative care | 8 weeks | Standard oncologic care | Baseline, 3 weeks, 8 weeks | EORTC QLQ-C30 |
| Temel et al., 2017 | America | Incurable lung or non-colorectal GI cancer diagnosed within 8 weeks | 175, 175 | 65.6, 64 | Model involving multiple settings: integrated early palliative care | At least once per month until death | Standard oncologic care | Baseline, 12 weeks, 24 weeks, 2, 4, 6 months before death | FACT-G, PHQ-9, HADS |
| Vanbutsele et al., 2018 |
Belgium | Advanced cancer, life expectancy of 12 months | 92, 94 | 64.5, 65 | Model involving multiple settings: integrated early palliative care | At least once per month until death | Standard oncologic care | Baseline, 12 weeks, 18 weeks, 24 weeks | EORTC QLQ-C30, MQOL, PHQ-9, HADS |
| Franciosi et al., 2019 | Italy | NSCLC, pancreatic, gastric or biliary tract cancer diagnosed within the previous 8 weeks, life expectancy greater than 3 months | 142, 139 | 68.5, 68 | Model involving multiple settings: integrated early palliative care | 6 months | Standard oncologic care | Baseline, 12 weeks | FACT-G, ECOG |
| Johnsen et al., 2019 | Denmark | Stage IV cancer according to the TNM classification or cancer in the central nervous system grade 3 or 4 | 145, 152 | – | Model involving multiple settings: integrated early palliative care | 8 weeks | Standard oncologic care | Baseline, 3 weeks, 8 weeks | EORTC QLQ-C30, HADS, FAMCARE-P16 |
| Nipp et al., 2020 | America | Incurable gastrointestinal or lung cancer diagnosed within 8 weeks, aged >64 years | 30, 32 | 73.9, 73.7 | Hospital outpatient model: integrated early palliative care | 2 in-person visits | Usual care | Baseline, 12 weeks | FACT-G, ESAS-r, GDS, ADLs, IADLs |
| El-Jawahri et al., 2021 | America | High-risk AML, received intensive chemotherapy | 86, 74 | 64.4, 64.4 | Inpatient consulting model: integrated early palliative care | At least twice per week during their initial and subsequent hospitalizations | Usual care | Baseline, 2 weeks, 4 weeks, 12 weeks, 24 weeks | FACT-Leukemia, HADS, PHQ-9, ESAS, PCL-C |
| Eychmüller et al., 2021 | Switzerland | Non-small cell lung, colorectal, castration-refractory prostate, breast cancer with visceral metastases, bladder and pancreatic cancer diagnosed within 16 weeks, not amenable or not responsive to curative treatment | 74, 76 | 67.3, 67.3 | Hospital outpatient model: integrated early palliative care | A 50 min structured conversation within 16 weeks of enrollment | Usual care | Baseline, 2 months, 4 months, 6 months | FACT-G, LSNS-6, DT |
Intervention strategies: structured multidisciplinary intervention: addressed the five domains of QoL including cognitive, physical, emotional, spiritual, and social functioning. FACT-G, Functional Assessment of Cancer Therapy-General; SDS, Symptoms Distress Scale; PHQ-9, Patient Health Questionnaire 9; ESDS, Enforced Social Dependency Scale; SF-12, 12-Item Short Form Survey; HADS, Hospital Anxiety and Depression Scale; MUIS, Mishel Uncertainty in Illness Scale; SEMCD-6, Self-Efficacy for Managing Chronic Disease Scale; FACT-BMT, FACT-Bone Marrow Transplant; ESAS, Edmonton Symptom Assessment System; PCL-C, Posttraumatic Stress Disorder Checklist Civilian Version; ECOG, Eastern Cooperative Oncology Group; EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life-C30 questionnaire; MQOL, McGill Quality of Life Questionnaire; FAMCARE, Family Satisfaction with Care; GDS, Geriatric Depression Scale; ADLs, activities of daily living; IADLs, Instrumental activities of daily living; LSNS-6, Lubben Social Network Scale; DT, single-item Distress Thermometer.
Risk of bias assessment
In all studies, there was a low or unclear risk of bias for most items (Figure S1), except for the presence of performance bias due to the lack of a double-blind design in seven studies. All trials were described as randomized, while 2 of the 12 (17%) did not describe the method used and were assessed as having an unclear risk. Allocation concealment was assessed as high risk in one trial (8%) and unclear risk in three trials (25%). Five trials (25%) were judged to have a high risk of attrition bias because more than 20% of participants dropped out. Attrition was caused by severe illness, exhaustion/weakness, hospital admission, transfer of care, death, failure to complete questionnaires and lack of interest. Nine trials (75%) had a protocol and were judged as having a low risk of selective reporting. Three trials (25%) were judged as having an unclear risk since the study protocols were not available and we did not have enough information in the study report to assess selective reporting.
QoL and psychological distress outcome measures
QoL was evaluated with different measures, including the European Organization for Research and Treatment of Cancer Quality of Life-C30 questionnaire (EORTC QLQ-C30) (27), and the Functional Assessment of Cancer Therapy-General (FACT-G) (28). Psychological distress was assessed with the Hospital Anxiety and Depression Scale (HADS) (29), Patient Health Questionnaire 9 (PHQ-9) (30), and PTSD Checklist–Civilian version (PCL-C) (31).
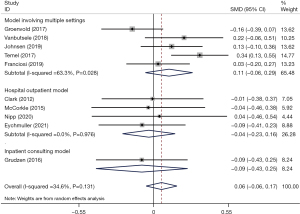
Effects on overall QoL
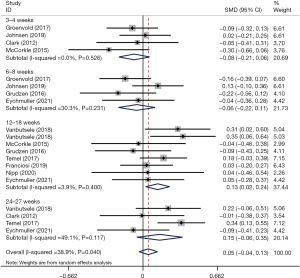
We determined the pooled effect size of IPC on QoL and compared it to the QoL of the control group in a random effects model. The overall effect size showed no significant difference between the two groups’ QoL scores (SMD =0.06; 95% CI: –0.06 to 0.17; P=0.318) (Figure 2). Pooled data were homogeneous (I2 = 34.6%, P=0.131). Furthermore, subgroup analyses were conducted to explore the impacts of different intervention models. In the subgroup analyses, a statistically significant difference was not found for the three different models versus conventional treatment (SMD multiple =0.11, P=0.204; SMD outpatient =−0.04, P=0.717; SMD inpatient =−0.09, P=0.611) (Figure 2). Sensitivity analysis was carried out by sequentially omitting each study. There was no alteration in the results, which indicated that our results were statistically reliable and robust. The sensitivity analysis is detailed in Figure S2. We further calculated whether the QoL score of the IPC group change differed at each time period in comparison to the conventional treatment group. At the 3–4 weeks and 6–8 weeks follow-ups, no significant difference in QoL was found between the groups (SMD =−0.08, 95% CI: −0.21 to 0.06, P=0.264; SMD =−0.06, 95% CI: −0.22 to 0.11, P=0.499). At the 12–18 weeks follow-up, there was a significant effect; there was a greater improvement in QoL in the intervention group than in the control group (SMD =0.13, 95% CI: 0.02 to 0.24, P=0.016). At the 26–27 weeks follow-up, the QoL score change did not differ significantly between the groups (SMD =0.15, 95% CI: −0.06 to 0.25, P=0.153) (Figure 3).
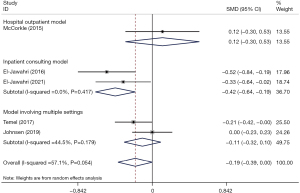
Effects on depression symptoms
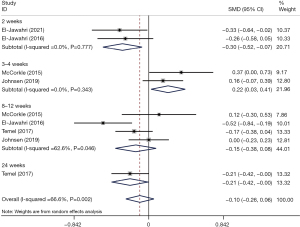
In the primary analysis, depression symptoms were lower in the IPC group than in the conventional care group, with marginal significance (SMD =−0.19, 95% CI: −0.39 to 0.00, P=0.053). Heterogeneity was moderate (I2 = 57.1%, P=0.054). The patient-involved inpatient consulting model showed a significant improvement in depressive symptoms (SMD =−0.42, 95% CI: −0.64 to −0.19, P<0.001) with respect to the other two models (Figure 4). The results of the sensitivity analysis showed that the overall effect size did not change very much when removing studies consecutively, which indicated that our results were statistically reliable and robust. The sensitivity analysis is detailed in Figure S3. Subgroup analyses were performed according to follow-up duration (Figure 5). A significant improvement in depressive symptoms was identified at the 2-week follow-up (SMD =−0.30, 95% CI: −0.52 to −0.07, P=0.009); however, the outcomes of IPC group indicated that the depressive symptoms were statistically greater at the 3–4-week follow-up (SMD =0.22, 95% CI: 0.03 to 0.41, P=0.026) than in the usual care group. At the 8–12 weeks follow-up, the change in depressive symptoms did not differ significantly between the groups (SMD =−0.15, 95% CI: −0.38 to 0.08, P=0.209).
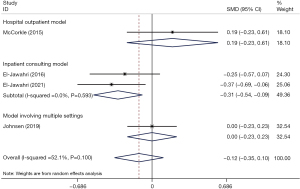
Effects on anxiety symptom
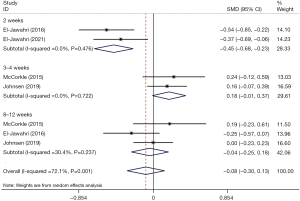
We compared the pooled effect size of IPC on anxiety symptoms to the control group in a random effects model. The overall analysis showed no significant difference between the two groups’ anxiety symptoms (SMD =−0.12; 95% CI: −0.35 to 0.10; P=0.295) (Figure 6). Heterogeneity was moderate (I2 = 52.1%, P=0.100). In the subgroup analyses of the three different models, a statistically significant difference was found for the inpatient consulting model versus conventional treatment (SMD =−0.31; 95% CI: −0.54 to −0.09; P=0.006) (Figure 6). According to the sensitivity analysis, the results remained stable and reliable (Figure S4). Furthermore, analyses were subgrouped by follow-up duration, and the IPC group had significantly fewer anxiety symptoms than the control group at the 2-week follow-up (SMD =−0.45, 95% CI: −0.68 to −0.23, P<0.001). At the 3–4 weeks and 8–12 weeks follow-ups, the change in anxiety symptoms did not differ significantly between the groups (SMD =0.18, 95% CI: −0.01 to 0.37, P=0.065; SMD =−0.04, 95% CI: −0.25 to 0.18, P=0.726) (Figure 7).
Effects on PTSD symptoms
Two studies reported the effect of IPC on PTSD symptoms. No significant heterogeneity was observed (I2 = 0%; P= 0.640). The results showed that PTSD symptoms were significantly reduced in the IPC group compared with the conventional group (SMD =−0.46, 95% CI: −0.69 to −0.23, P<0.001) (Figure 8). Only two studies were included, and we did not perform subgroup analysis.

Risk of publication bias across studies
Egger’s test was performed to evaluate the publication bias of the included studies. The funnel plot was symmetric for both QoL and psychological distress outcomes (Figure S5-S7), showing a lack of publication bias (P=0.309; P=0.852; P=0.962).
Discussion
In this meta-analysis, we assessed and synthesized clinical trial evidence of the effects of IPC on QoL and psychological distress outcomes in patients with advanced cancer. Our results suggested no significant difference between the three different models of IPC and conventional treatment on overall QoL. However, there was a long-lasting favorable effect of IPC on overall QoL throughout the follow-up time period of 12 to 18 weeks. There is an indication that the inpatient consulting model was more effective than the other models in reducing depression and anxiety symptoms. In the early follow-up period of approximately 2 weeks, IPC was shown to be significantly more effective on these symptoms. IPC was also effective in decreasing PTSD symptoms.
No significant differences in overall QoL were observed between IPC and conventional treatment. This finding was not consistent with previous meta-analysis results (32). However, we performed a more comprehensive systematic analysis and included the latest studies that the previous meta-analysis did not include. As is the case in most research settings of palliative care, the included trials differed largely in several aspects, such as the population studied, the outcomes chosen, the clinical setting, and the duration of the study. The true effect may be substantially different. Even when we separately examined the efficacy of the three models of IPC in our analysis, we did not find a significant difference in efficacy. Fulton et al. reported that there was no significant difference in patients’ psychological distress between integrated outpatient palliative care treatment and conventional treatment (8). These results are consistent with our findings. Apart from that, we also found that the inpatient consulting model of IPC has a desirable effect on psychological distress. The mechanisms underlying these reductions in psychological distress in patients receiving inpatient palliative care are not completely clear. Palliative care may improve psychological conditions by providing patients with the skills to cope effectively with life-threatening illness (33). We speculate that it is plausible that inpatient palliative care is more effective for enhancing patients’ adaptive coping strategies (34). Future work should examine whether patients’ coping skills mediate the effect of different integration models of palliative care intervention on psychological distress in patients with advanced cancer.
Some evidence suggests that for the optimal therapeutic benefit of palliative care to be realized, continuity of intervention by a multidisciplinary team is needed for at least 3–4 months (35). This is confirmed by our results of long-term improvements (at the 12–18 weeks follow-up) in overall QoL. Our results regarding psychological distress showed that the IPC intervention for depression and anxiety symptoms was most effective at an early stage of approximately 2 weeks. Multidisciplinary palliative care teams frequently focus on coping strategies and managing expectations, which could potentially explain the psychological improvement (25). However, there was no difference in the long-term improvement of psychological distress between IPC and conventional care in patients with advanced cancer. Given the high mortality and disease burden among this population, possible confounders (e.g., health care utilization and end-of-life outcomes) should be comprehensively considered in future work.
This meta-analysis provides high-quality evidence that IPC are potentially effective in improving QoL and relieving psychological distress for advanced cancer patients. Compared with previous meta-analyses, this meta-analysis explored these outcomes according to various types of IPC but also according to the follow-up time/period. Our meta-analysis was conducted as a Cochrane review following the instructions from the Cochrane Handbook and the PRISMA guidelines.
Study limitations
There are several limitations to the present meta-analysis that should be addressed prior to its application in clinical practice. Between-study heterogeneity persisted in some of the subgroups, suggesting the presence of other potentially confounding factors, resulting from differences in the intervention models, length of intervention, diversity in sample sizes, patient ages, cancer types, and other factors. In addition, it was difficult to include more patients with different types of cancer because retrospective cohorts and observational studies did not meet our inclusion criteria. RCTs provide high-level evidences but are sometimes not feasible due to ethical issues, substantial costs, and inadequate duration of follow-up. Thus, a more comprehensive meta-analysis is needed with more cases. Furthermore, we did not have access to sufficient data to determine whether IPC increased all dimensions of the patients’ QoL. Notwithstanding these limitations, the current study provides important evidence suggesting the efficacy of IPC for patients with advanced cancer in improving overall QoL and reducing psychological distress.
Clinical implications
This meta-analysis has implications for both research and clinical practice. It provides a comprehensive overview of the available randomized evidence on the effectiveness of IPC treatment for emotional distress and QoL in patients with advanced cancer. We had a specific focus on various integration models and the long-term efficacy of IPC. Our results may imply that the inpatient consultation model is more favorable for reducing depression and anxiety symptoms, especially in the early period of approximately 2 weeks. This may assist in guiding clinicians in making early treatment decisions in clinical practice. However, our analyses are based on reports of depressive and anxiety symptoms, not diagnoses of disorders. It will be important in future research to determine whether IPC decreases the risk for mood or anxiety disorders in patients with advanced cancer. Furthermore, future studies should use a consensus-based measure of QoL that assesses as many domains as possible (physical, psychosocial, spiritual). Finally, our results highlight the need for further research assessing long-term real-world data on the psychological profiles of patients receiving different versions of IPC as well as the effects on QoL. These observations should guide future research and clinical practice.
Conclusions
Our results suggest that IPC can effectively improve the QoL and alleviate early psychological distress of patients with advanced cancer. There is an indication that the inpatient consulting model was more effective than other models in reducing depression and anxiety symptoms. These results were based on randomized clinical trial studies and require further verification.
Acknowledgments
Funding: This study was supported by National Natural Science Foundation of China (No. 81301150), Hebei Province Natural Science Foundation of China (No. H2018206034), Introduce Foreign Intellectual Projects of Finance Department in Hebei Province (No. YZ201801) and Medical Science Key Research Program from Department of Health of Hebei Province (No. 20210417).
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-162/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-162/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Latest global cancer data: Cancer burden rises to 19.3 million new cases and 10.0 million cancer deaths in 2020. Available online: https://www.iarc.who.int/news-events/latest-global-cancer-data-cancer-burden-rises-to-19-3-million-new-cases-and-10-0-million-cancer-deaths-in-2020/
- Skov Benthien K, Adsersen M, Petersen MA, et al. Is specialized palliative cancer care associated with use of antineoplastic treatment at the end of life? A population-based cohort study. Palliat Med 2018;32:1509-17. [Crossref] [PubMed]
- Bakitas MA, Tosteson TD, Li Z, et al. Early Versus Delayed Initiation of Concurrent Palliative Oncology Care: Patient Outcomes in the ENABLE III Randomized Controlled Trial. J Clin Oncol 2015;33:1438-45. [Crossref] [PubMed]
- Heywood R, McCarthy AL, Skinner TL. Efficacy of Exercise Interventions in Patients With Advanced Cancer: A Systematic Review. Arch Phys Med Rehabil 2018;99:2595-620. [Crossref] [PubMed]
- Shin J, Temel J. Integrating palliative care: when and how? Curr Opin Pulm Med 2013;19:344-9. [PubMed]
- Greer JA, Jackson VA, Meier DE, et al. Early integration of palliative care services with standard oncology care for patients with advanced cancer. CA Cancer J Clin 2013;63:349-63. [Crossref] [PubMed]
- Ferrell BR, Temel JS, Temin S, et al. Integration of Palliative Care Into Standard Oncology Care: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2017;35:96-112. [Crossref] [PubMed]
- Fulton JJ, LeBlanc TW, Cutson TM, et al. Integrated outpatient palliative care for patients with advanced cancer: A systematic review and meta-analysis. Palliat Med 2019;33:123-34. [Crossref] [PubMed]
- Oluyase AO, Higginson IJ, Yi D, et al. Health Services and Delivery Research, in Hospital-based specialist palliative care compared with usual care for adults with advanced illness and their caregivers: a systematic review. Southampton (UK): NIHR Journals Library, 2021.
- Hoerger M, Wayser GR, Schwing G, et al. Impact of Interdisciplinary Outpatient Specialty Palliative Care on Survival and Quality of Life in Adults With Advanced Cancer: A Meta-Analysis of Randomized Controlled Trials. Ann Behav Med 2019;53:674-85. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264-9, W64.
- Lynn J, Adamson DM. Living Well at the End of Life: Adapting Health Care to Serious Chronic Illness in Old Age. 2003. Available online: https://apps.dtic.mil/sti/pdfs/ADA416211.pdf
- Higgins JP. Cochrane handbook for systematic reviews of interventions version 5.0. 1. The Cochrane Collaboration. Available online: http://www.cochrane-handbook.org
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Clark MM, Rummans TA, Atherton PJ, et al. Randomized controlled trial of maintaining quality of life during radiotherapy for advanced cancer. Cancer 2013;119:880-7. [Crossref] [PubMed]
- McCorkle R, Jeon S, Ercolano E, et al. An Advanced Practice Nurse Coordinated Multidisciplinary Intervention for Patients with Late-Stage Cancer: A Cluster Randomized Trial. J Palliat Med 2015;18:962-9. [Crossref] [PubMed]
- Grudzen CR, Richardson LD, Johnson PN, et al. Emergency Department-Initiated Palliative Care in Advanced Cancer: A Randomized Clinical Trial. JAMA Oncol 2016;2:591-8. [Crossref] [PubMed]
- Groenvold M, Petersen MA, Damkier A, et al. Randomised clinical trial of early specialist palliative care plus standard care versus standard care alone in patients with advanced cancer: The Danish Palliative Care Trial. Palliat Med 2017;31:814-24. [Crossref] [PubMed]
- Temel JS, Greer JA, El-Jawahri A, et al. Effects of Early Integrated Palliative Care in Patients With Lung and GI Cancer: A Randomized Clinical Trial. J Clin Oncol 2017;35:834-41. [Crossref] [PubMed]
- Vanbutsele G, Pardon K, Van Belle S, et al. Effect of early and systematic integration of palliative care in patients with advanced cancer: a randomised controlled trial. Lancet Oncol 2018;19:394-404. [Crossref] [PubMed]
- Franciosi V, Maglietta G, Degli Esposti C, et al. Early palliative care and quality of life of advanced cancer patients-a multicenter randomized clinical trial. Ann Palliat Med 2019;8:381-9. [Crossref] [PubMed]
- Johnsen AT, Petersen MA, Sjøgren P, et al. Exploratory analyses of the Danish Palliative Care Trial (DanPaCT): a randomized trial of early specialized palliative care plus standard care versus standard care in advanced cancer patients. Support Care Cancer 2020;28:2145-55. [Crossref] [PubMed]
- Eychmüller S, Zwahlen S, Fliedner MC, et al. Single early palliative care intervention added to usual oncology care for patients with advanced cancer: A randomized controlled trial (SENS Trial). Palliat Med 2021;35:1108-17. [Crossref] [PubMed]
- Nipp RD, Temel B, Fuh CX, et al. Pilot Randomized Trial of a Transdisciplinary Geriatric and Palliative Care Intervention for Older Adults With Cancer. J Natl Compr Canc Netw 2020;18:591-8. [Crossref] [PubMed]
- El-Jawahri A, LeBlanc T, VanDusen H, et al. Effect of Inpatient Palliative Care on Quality of Life 2 Weeks After Hematopoietic Stem Cell Transplantation: A Randomized Clinical Trial. JAMA 2016;316:2094-103. [Crossref] [PubMed]
- El-Jawahri A, LeBlanc TW, Kavanaugh A, et al. Effectiveness of Integrated Palliative and Oncology Care for Patients With Acute Myeloid Leukemia: A Randomized Clinical Trial. JAMA Oncol 2021;7:238-45. [Crossref] [PubMed]
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365-76. [Crossref] [PubMed]
- Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 1993;11:570-9. [Crossref] [PubMed]
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. [Crossref] [PubMed]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13. [Crossref] [PubMed]
- Smith MY, Redd W, DuHamel K, et al. Validation of the PTSD Checklist-Civilian Version in survivors of bone marrow transplantation. J Trauma Stress 1999;12:485-99. [Crossref] [PubMed]
- Gaertner J, Siemens W, Meerpohl JJ, et al. Effect of specialist palliative care services on quality of life in adults with advanced incurable illness in hospital, hospice, or community settings: systematic review and meta-analysis. BMJ 2017;357:j2925. [Crossref] [PubMed]
- Greer JA, Jacobs JM, El-Jawahri A, et al. Role of Patient Coping Strategies in Understanding the Effects of Early Palliative Care on Quality of Life and Mood. J Clin Oncol 2018;36:53-60. [Crossref] [PubMed]
- Amano K, Hopkinson J, Baracos V. Psychological symptoms of illness and emotional distress in advanced cancer cachexia. Curr Opin Clin Nutr Metab Care 2021; [Epub ahead of print]. [Crossref] [PubMed]
- Davis MP, Temel JS, Balboni T, et al. A review of the trials which examine early integration of outpatient and home palliative care for patients with serious illnesses. Ann Palliat Med 2015;4:99-121. [PubMed]



