
Effects of low-dose intravenous immunoglobulin as the adjunctive therapy in septic shock patients with and without hypogammaglobulinemia: a retrospective cohort study
Introduction
Sepsis and sepsis-related multiple organ failure are major causes of mortality in the intensive care unit (ICU) (1,2). In addition to antimicrobial treatment, the goals of septic shock treatment include regulation of immune function and removal of pathogens from the circulation (3,4). Intravenous immunoglobulin (IVIG) therapy performs several functions in the treatment of pathogenic infections: (I) opsonization, (II) complement activation, (III) toxin and virus neutralization, (IV) anti-inflammatory cytokine modulation, and (V) antibody-dependent cell-mediated cytotoxicity. However, evidence for the ability of IVIG administration to improve outcomes is considered insufficient based on the results of the SBITS study (5), a large-scale randomized control trial (RCT), and IVIG therapy is not recommended in sepsis guidelines [the Society of Critical Care Medicine (SCCM)/the European Society of Intensive Care Medicine, and the Japanese Society of Intensive Care Medicine/the Japanese Association for Acute Medicine (JAAM)] (6,7).
Serum immunoglobulin G (IgG) levels decline in the early phase of sepsis due to suppressed production, leakage from vessels, and increased consumption (8,9), and low IgG levels at ICU entry are associated with poor prognoses (10-13). In Japan, all patients with septic shock admitted to the ICU are treated according to sepsis guidelines (6,7) and, in addition, receive low-dose IVIG as adjunctive therapy (5 g/day for 3 days, total of 15 g or 0.3 g/kg) (14), regardless of serum IgG levels at the time of diagnosis of septic shock. The IVIG dose for this regimen, which is lower than the global standard dose (0.9 g/kg) (5), has been reported to have efficacy as adjuvant therapy in sepsis (15,16). Current guidelines (6,7) do not recommend IVIG use as a routine part of the management of sepsis, however this recommendation is not specifically formulated for septic patients with hypogammaglobulinemia. It is not reported whether the presence of hypogammaglobulinemia require IVIG administration in septic shock patients. Here we thought to investigate whether serum IgG levels can serve as a biomarker to predict the efficacy of IVIG treatment for septic shock. Initial levels of serum IgG might help to identify those patients who might benefit from an adjunctive IVIG treatment. The purpose of this study was to retrospectively compare the efficacy of low-dose IVIG as an adjunctive therapy in septic shock patients with and without hypogammaglobulinemia by dividing them into two groups based on their median serum IgG level before IVIG administration of 829 mg/dL. We present the following article in accordance with the STROBE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-21-3694/rc).
Methods
Subjects
Patients who received low-dose IVIG (5 g/day for 3 days) as adjuvant therapy for septic shock in a medical facility under Japan’s health insurance system and who were admitted to the Oita University Hospital ICU between January 2015 and December 2017 were enrolled in this retrospective study. Septic shock was defined according to the diagnostic criteria of the American College of Chest Physicians (ACCP)/SCCM guidelines (17). Clinical parameters to identify patients with septic shock are: vasopressor requirement to maintain a mean arterial pressure of 65 mmHg or greater and serum lactate level greater than 2 mmol/L (>18 mg/dL) in the absence of hypovolemia (17). Medical treatment based on the sepsis guidelines (6,7) was provided to all patients. Exclusion criteria were as follows: patients under 18 years old, patients who withdrew within 48 hours of ICU entry, patients who received IVIG before ICU admission, and patients whose serum IgG levels were unknown (Table 1). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethical Review Board of Oita University Faculty of Medicine (approval number: 691) and individual consent for this retrospective analysis was waived. We received no specific grant from any funding agency.
Table 1
| Variables | L group (n=40) | H group (n=40) | Total (n=80) |
|---|---|---|---|
| Pre-serum IgG (mg/dL) | 590 [464–679] | 1,082 [934–1,230]* | 829 [590–1,082] |
| Total IVIG dose (g/kg) | 0.29 (0.24–0.33) | 0.28 (0.27–0.33) | 0.29 (0.25–0.33) |
| Sex (male/female) | 23/17 | 26/14 | 49/31 |
| Age (years) | 73 [60–79] | 68 [56–79] | 69 [59–79] |
| Body weight (kg) | 52 [46–64] | 53 [45–56] | 52[45–61] |
| APACHE II score | 22 [18–28] | 23 [17–28] | 22 [17–28] |
| SOFA score | 9 [8–10] | 10 [8–12] | 9 [8–11] |
| JAAM DIC score | 4 [2–5] | 4 [2–6] | 4 [2–5] |
| Serum PCT (ng/mL) | 9.2 (2.8–35.7) | 9.6 (5.4–25.8) | 9.6 (4.4–29.8) |
| Types of nosocomial infections | |||
| Surgical | 31 (77.5%) | 32 (80.0%) | 63 (78.8%) |
| Medical | 9 (22.5%) | 8 (20.0%) | 17 (21.2%) |
| Blood culture positive | 16 (40.0%) | 19 (47.5%) | 35 (43.8%) |
| Gram (+) bacteria | 8 (20.0%) | 12 (30.0%) | 20 (25.0%) |
| Gram (−) bacteria | 7 (17.5%) | 7 (17.5%) | 14 (17.5%) |
| Fungus | 1 (2.5%) | 0 | 1 (1.3%) |
| Site of infection | |||
| Respiratory | 13 (32.5%) | 11 (27.5%) | 24 (30.0%) |
| Intra-abdominal or retroabdominal, or pelvic cavity | 13 (32.5%) | 11 (27.5%) | 24 (30.0%) |
| Blood or catheter | 3 (7.5%) | 6 (15.0%) | 9 (11.3%) |
| Soft tissue or bone | 4 (10.0%) | 2 (5.0%) | 6 (7.5%) |
| Urinary | 2 (5.0%) | 3 (7.5%) | 5 (6.3%) |
| Others | 1 (2.5%) | 2 (5.0%) | 3 (3.8%) |
| Unknown | 4 (10.0%) | 5 (12.5%) | 9 (11.3%) |
| Use of catecholamines | 40 (100%) | 40 (100%) | 80 (100%) |
| Use of artificial ventilation | 36 (90.0%) | 36 (90.0%) | 72 (90.0%) |
| Initiative antibiotics | |||
| Administering antibiotics within 3 hours of ICU | 40 (100%) | 40 (100%) | 80 (100%) |
| Initial use of 2 or more antibiotics | 20 (50.0%) | 22 (55.0%) | 42 (52.5%) |
| Supportive therapy | |||
| Early enteral nutrition | 31 (77.5%) | 30 (75.0%) | 61 (76.3%) |
| Continuous renal replacement therapy | 28 (70.0%) | 27 (67.5%) | 55 (68.8%) |
| Use of AT III concentrate | 28 (70.0%) | 31 (77.5%) | 59 (73.8%) |
| Use of rTM agent | 25 (62.5%) | 27 (67.5%) | 52 (65.0%) |
Criteria of septic shock: vasopressor requirement to maintain a mean arterial pressure of 65 mmHg or greater and serum lactate level greater than 2 mmol/L (>18 mg/dL) in the absence of hypovolemia. Continuous variables were presented as median and 25–75% IQR. At ICU entry, the patients who were diagnosed with septic shock and met the inclusion criteria were divided into two groups: a low group with serum IgG levels <829 mg/dL (low-normal range) (L group) and a high group with serum IgG levels ≥829 mg/dL (H group). Categorical variables were presented as number (%). *, P<0.05 vs. L group (Mann-Whitney U-test). IgG, immunoglobulin G; IVIG, intravenous immunoglobulin; APACHE, Acute Physiology and Chronic Health Evaluation; SOFA, Sequential Organ Failure Assessment; JAAM, Japanese Association for Acute Medicine; DIC, Disseminated Intravascular Coagulation; PCT, procalcitonin; AT III, antithrombin III; rTM, recombinant thrombomodulin; IQR, interquartile ratio.
Assessment of serum IgG level
Patients’ demographic and laboratory data were collected using electronic medical charts and included: (I) serum IgG level (mg/dL) before IVIG administration, (II) total IVIG dosage (g/kg), (III) age, sex, and body weight, (IV) Acute Physiology and Chronic Health Evaluation (APACHE) II score (18), (V) Sequential Organ Failure Assessment (SOFA) score (19), (VI) acute Disseminated Intravascular Coagulation (DIC) score (20), (VII) serum procalcitonin (ng/mL), (VIII) blood cultures, (IX) types of nosocomial infections, (X) site of infection, (XI) use of catecholamines, (XII) use of artificial ventilation, (XIII) antimicrobial agent administered within three hours after ICU entry, (XIV) combination of antimicrobial agents administered, (XV) early stage enteral feeding within 48 hours after ICU entry, (XVI) continuous renal replacement treatment, and (XVII) anti-DIC treatment [preparation of antithrombin III (AT III) and recombinant thrombomodulin (rTM)]. At ICU entry, the patients who were diagnosed with septic shock and met the inclusion criteria were divided into two groups: a low group with serum IgG levels <829 mg/dL (low-normal range) (L group) and a high group with serum IgG levels ≥829 mg/dL (H group).
Effect of low-dose IVIG administration on serum IgG levels
Serum IgG levels after low-dose IVIG administration in the L group and the H group were compared. The primary outcome assessed was 28-day survival probability. Secondary outcomes were the durations of artificial ventilation and ICU stays.
Statistical analysis
Data were presented as median and interquartile ratio (IQR) or numbers, as appropriate. Continuous and categorical variables were compared using the Wilcoxon or Mann-Whitney U test. Survival probabilities were compared using the Kaplan-Meier method and the log-rank test. Logistic regression models were used to explore the influence of independent variables on the clinical outcomes as the dependent variable. Statistical analysis was carried out with Statflex Statistical Software version 6.0 (Artech Co. Ltd., Osaka, Japan). A two-sided P <0.05 was considered statistically significant. A planned sample size of 65 patients with all examination was estimated to provide 80% power to detect a 10% differences in 28-day survival probability (90% to 80%) in two groups with and without hypogammaglobulinemia using a two-sided type-I error of 5%.
Results
Patient characteristics
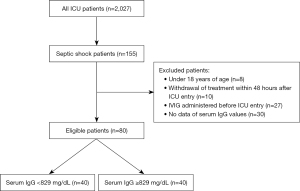
A total of 2,027 patients were admitted to the ICU during the study period; 155 of these had septic shock. Excluded patients were as follows: aged less than 18 years (8 patients), withdrew within 48 hours after ICU entry (10 patients), IVIG administered prior to ICU entry (27 patients), and no available data for serum IgG values (30 patients). Thus, a total of 80 patients were enrolled in the study, 40 in the L group and 40 in the H group (Figure 1). Patient characteristics are shown in Table 1. Mean serum IgG levels were 590 mg/dL (464–679 mg/dL) and 1,082 mg/dL (934–1,230 mg/dL) in the L group and the H group, respectively. Total IVIG doses were 0.29 g/kg (0.24–0.33 g/kg) and 0.28 g/kg (0.27–0.33 g/kg) in the L group and the H group, respectively. No significant differences were observed in age, sex, weight, APACHE II score, SOFA score, acute DIC score, serum procalcitonin level, types of nosocomial infections, use of catecholamines, or use of artificial ventilation between the two groups. Blood culture positivity rates were 40.0% and 47.5% in the L and H groups, respectively. Gram-positive bacteria were the most commonly detected in both groups. Patients in the L group had respiratory infections, while infections of the intra-abdominal, retroabdominal, or pelvic cavities, as well as respiratory infections, were found in the H group. Antimicrobial agents were administered in all patients within 3 hours after admission to the ICU. Empirical therapy by two or more kinds of antibiotics was performed in approximately 50% of patients in both groups. There were no significant differences in early enteral feeding, continuous renal replacement therapy, and anti-DIC treatment [preparation of AT III and rTM] between the groups.
Serum IgG levels before and after administration of IVIG
Table 2 shows serum IgG levels before and after IVIG administration. In the L group, serum IgG levels significantly increased to 962 mg/dL (852–1,174 mg/dL) after administration of IVIG compared to initial values [590 mg/dL (464–679 mg/dL)]. Serum IgG levels also increased significantly in the H group, rising to 1,331 mg/dL (1,232–1,515 mg/dL) after administration of IVIG from initial values of 1,082 mg/dL (934–1,230 mg/dL). Serum IgG levels in the H group were significantly higher than in the L group both before and after administration of IVIG.
Table 2
| Variables | L group (n=40) | H group (n=40) | |||
|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||
| Serum IgG (mg/dL) | 590 [464–679] | 962 [852–1,174]* | 1,082 [934–1,230]# | 1,331 [1,232–1,515]*# | |
At ICU entry, the patients who were diagnosed with septic shock and met the inclusion criteria were divided into two groups: a low group with serum IgG levels <829 mg/dL (low-normal range) (L group) and a high group with serum IgG levels ≥829 mg/dL (H group). Data were presented as median and 25–75% IQR. *, P<0.05 vs. Pre (Wilcoxon test); #, P<0.05 vs. L group (Mann-Whitney U test). IVIG, intravenous immunoglobulin; IgG, immunoglobulin G; IQR, interquartile ratio.
Effects of IVIG administration on 28-day survival probability, the duration of artificial ventilation and ICU stays
Figure 2 shows 28-day survival probabilities after IVIG administration. No significant differences were observed between the L group (85.0%) and the H group (90.0%) (log-rank test: P=0.457). All patients were followed up to the death.
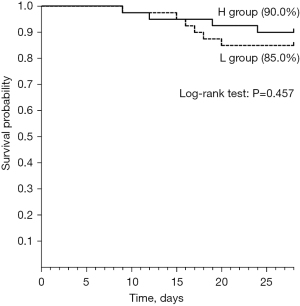
Table 3 shows the duration of artificial ventilation and ICU stays after IVIG administration in the L group and the H group. No significant differences were observed.
Table 3
| Variables | L group (n=40) | H group (n=40) |
|---|---|---|
| The length of artificial ventilation (days) | 9 [4–13] | 6 [4–10] |
| The length of ICU stay (days) | 12 [7–16] | 10 [7–12] |
At ICU entry, the patients who were diagnosed with septic shock and met the inclusion criteria were divided into two groups: a low group with serum IgG levels <829 mg/dL (low-normal range) (L group) and a high group with serum IgG levels ≥829 mg/dL (H group). Data were presented as median and 25–75% IQR. IVIG, intravenous immunoglobulin; ICU, intensive care unit; IQR, interquartile ratio.
Possible influencing factors in the hypogammaglobulinemia-outcomes association, such as the antibiotics, were assessed using logistic regression modeling (Table 4). In multivariate analysis, 28-day survival, the duration of artificial ventilation and ICU stays, and the main antibiotics (carbapenem, anti-MRSA and anti-fungal) were not associated with serum IgG levels.
Table 4
| Variables | OR (95% CI) | P values |
|---|---|---|
| 28-day survival (days) | 1.0 (0.877–1.150) | 0.957 |
| The length of artificial ventilation (days) | 1.02 (0.839–1.250) | 0.814 |
| The length of ICU stay (days) | 0.968 (0.783–1.200) | 0.767 |
| Initiative antibiotics (carbapenem) | 0.443 (0.034–5.660) | 0.531 |
| Initiative antibiotics (anti-MRSA) | 1.580 (0.486–5.120) | 0.449 |
| Initiative antibiotics (anti-fungal) | 0.804 (0.237–2.730) | 0.726 |
In the multivariable adjusted models, all dependent variables were adjusted for each other. IgG, immunoglobulin G; OR, odds ratio; CI, confidence interval; ICU, intensive care unit; MRSA, methicillin-resistant Staphylococcus aureus.
Discussion
Low-dose IVIG administration as adjuvant therapy had positive effects regardless of hypogammaglobulinemia in septic shock patients. The median serum IgG level in septic shock patients at ICU entry was 829 mg/dL, which is low compared to the mean serum IgG level reported in 840 healthy Japanese adults (1,414 mg/dL) (21) but comparable to data from Ishikura et al. (16), who reported 854 mg/dL serum IgG in sepsis patients at ICU entry before IVIG administration.
In this study, enrolled patients were divided into two groups: a low group with serum IgG <829 mg/dL (lower normal range) (L group) and a high group with serum IgG level ≥829 mg/dL (H group). Patients in the L group had hypogammaglobulinemia before IVIG administration because the serum IgG level was 590 mg/dL (464–679 mg/dL). Hypogammaglobulinemia is generally defined as a serum IgG concentration below 870 mg/dL. Therefore, patients in our L group were considered to be hypogammaglobulinemia. There are several reports that hypogammaglobulinemia at diagnosis is associated with poor prognosis in septic shock patients (10-13). In a study by Taccone et al. (10), prognosis was worse in septic shock patients with serum IgG levels below 650 mg/dL. Tamayo et al. (11) reported serum IgG levels of 829 mg/dL in a survivor group, compared with 598 mg/dL in a non-survivor group of septic shock patients. Prucha et al. (12) reported significantly higher mortality in septic shock patients with hypogammaglobulinemia (<600 mg/dL serum IgG) (45.5% vs. 38.2% in patients with ≥600 mg/dL serum IgG). In our study, the 28-day mortality rate in patients with hypogammaglobulinemia (15.0%) was significantly lower than those reported in other studies and was not significantly different from that in the high-IgG group (10.0%). Therefore, low-dose IVIG administration may improve 28-day mortality in septic shock, regardless of serum IgG levels. Akatsuka et al. (13) reported low IgG levels (<670 mg/dL) in critically ill patients are associated with poor clinical outcomes related to 28-day mortality.
The low-dose adjuvant IVIG regimen for severe infection covered by Japanese health insurance is 5 g/day for three days (14-16). Here, we report that this intervention significantly increased serum IgG levels from 590 mg/dL (464–679 mg/dL) to 962 mg/dL (852–1,174 mg/dL) in the L group and from 1,082 mg/dL (934–1,230 mg/dL) to 1,331 mg/dL (1,232–1,515 mg/dL) in the H group. This represents increases in serum IgG concentrations from levels comparable to those in non-surviving patients in previous reports (10-13) to levels observed in the surviving patients. The SBITS study (5), which is the only large-scale RCT of IVIG and septic shock, was conducted in 2007, and subjects were enrolled between 1991 and 1995, before implementation of the sepsis guidelines. In a subgroup analysis of the SBITS study, Dietz et al. (22) divided enrolled patients into four groups based on serum IgG levels: (I) IgG ≤610 mg/dL, (II) 610 < IgG ≤ 840 mg/dL, (III) 840 < IgG ≤ 1,190 mg/dL, and (IV) IgG >1,190mg/dL and assessed prognosis in patients with or without hypogammaglobulinemia. They reported no association between hypogammaglobulinemia and worse survival; rather, only patients with hypergammaglobulinemia (serum IgG concentration of 1,190 mg/dL or higher) had worse survival rates. Hence, they suggested that IVIG in septic patients with hypergammaglobulinemia may cause an excessive immune response and worsen prognosis. In our study, patients were treated according to the guidelines for the treatment of sepsis, and a lower dose (0.3 g/kg) of IVIG than the global standard dose (0.9 g/kg) was added as adjunctive therapy. The 28-day survival probability was not significantly different between the H group (90.0%) and the L group (85.0%). Therefore, a low dose of IVIG may have a positive adjuvant effect in the treatment of septic shock without triggering an excessive immune response in patients with hypergammaglobulinemia. However, since we have no comparative data with a no-IVIG group or a global standard dose (0.9 g/kg) group, this will require further investigation.
The lack of laboratory data to evaluate efficacy of IVIG therapy in patients with or without hypogammaglobulinemia, such as serum IgG, is a concern when using the IVIG. In the present study, however, none of patients lack the laboratory data.
The limitations of this study are as follows: (I) this was a single-center, retrospective study with a limited number of patients; further prospective multicenter collaborative studies are needed. (II) The median serum IgG level of 829 mg/dL was used as a criterion to classify and compare only two groups, but our analysis may have benefited from further subdivision into several groups with narrower serum IgG ranges. (III) The study was conducted only with the dose indicated by insurance in Japan (0.3 g/kg) and not with a higher IVIG dose, such as the world standard dose (0.9 g/kg). (IV) IVIG treated group is not compared with IVIG non-treated group. At present, it is not clear wherever IVIG therapy have efficacy for the septic shock patients. Further controlled studies are needed to confirm the efficacy of IVIG therapy, but ethical setting is difficult because the prognosis may be worse in the control group (IVIG non-treated group).
Conclusions
Serum IgG levels was not associated with the clinical outcomes by low-dose IVIG treatment. The present study found that the prognosis by low-dose IVIG therapy as adjuvant therapy for septic shock might be no different with and without hypogammaglobulinemia.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-21-3694/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-21-3694/dss
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-21-3694/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-21-3694/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethical Review Board of Oita University Faculty of Medicine (approval number: 691) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fleischmann C, Scherag A, Adhikari NK, et al. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am J Respir Crit Care Med 2016;193:259-72. [Crossref] [PubMed]
- Abe T, Ogura H, Shiraishi A, et al. Characteristics, management, and in-hospital mortality among patients with severe sepsis in intensive care units in Japan: the FORECAST study. Crit Care 2018;22:322. [Crossref] [PubMed]
- Negi VS, Elluru S, Sibéril S, et al. Intravenous immunoglobulin: an update on the clinical use and mechanisms of action. J Clin Immunol 2007;27:233-45. [Crossref] [PubMed]
- Nimmerjahn F, Ravetch JV. Anti-inflammatory actions of intravenous immunoglobulin. Annu Rev Immunol 2008;26:513-33. [Crossref] [PubMed]
- Werdan K, Pilz G, Bujdoso O, et al. Score-based immunoglobulin G therapy of patients with sepsis: the SBITS study. Crit Care Med 2007;35:2693-701. [PubMed]
- Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med 2021;47:1181-247. [Crossref] [PubMed]
- Egi M, Ogura H, Yatabe T, et al. The Japanese Clinical Practice Guidelines for Management of Sepsis and Septic Shock 2020 (J-SSCG 2020). J Intensive Care 2021;9:53. [Crossref] [PubMed]
- Almansa R, Tamayo E, Andaluz-Ojeda D, et al. The original sins of clinical trials with intravenous immunoglobulins in sepsis. Crit Care 2015;19:90. [Crossref] [PubMed]
- Venet F, Gebeile R, Bancel J, et al. Assessment of plasmatic immunoglobulin G, A and M levels in septic shock patients. Int Immunopharmacol 2011;11:2086-90. [Crossref] [PubMed]
- Taccone FS, Stordeur P, De Backer D, et al. Gamma-globulin levels in patients with community-acquired septic shock. Shock 2009;32:379-85. [Crossref] [PubMed]
- Tamayo E, Fernández A, Almansa R, et al. Beneficial role of endogenous immunoglobulin subclasses and isotypes in septic shock. J Crit Care 2012;27:616-22. [Crossref] [PubMed]
- Prucha M, Zazula R, Herold I, et al. Presence of hypogammaglobulinemia in patients with severe sepsis, septic shock, and SIRS is associated with increased mortality. J Infect 2014;68:297-9. [Crossref] [PubMed]
- Akatsuka M, Tatsumi H, Sonoda T, et al. Low immunoglobulin G level is associated with poor outcomes in patients with sepsis and septic shock. J Microbiol Immunol Infect 2021;54:728-32. [Crossref] [PubMed]
- Nakamura K, Inokuchi R, Fukushima K, et al. Single versus divided administration of intravenous immunoglobulin for sepsis: a retrospective and historical control study. Minerva Anestesiol 2019;85:156-63. [Crossref] [PubMed]
- Masaoka T, Hasegawa H, Takaku F, et al. The efficacy of intravenous immunoglobulin in combination therapy with antibiotics for severe infections. Jpn J chemother 2000;48:199-217.
- Ishikura H, Nakamura Y, Kawano Y, et al. Intravenous immunoglobulin improves sepsis-induced coagulopathy: A retrospective, single-center observational study. J Crit Care 2015;30:579-83. [Crossref] [PubMed]
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992;20:864-74. [Crossref] [PubMed]
- Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818-29. [Crossref] [PubMed]
- Vincent JL, de Mendonça A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on "sepsis-related problems" of the European Society of Intensive Care Medicine. Crit Care Med 1998;26:1793-800. [Crossref] [PubMed]
- Gando S, Saitoh D, Ogura H, et al. Natural history of disseminated intravascular coagulation diagnosed based on the newly established diagnostic criteria for critically ill patients: results of a multicenter, prospective survey. Crit Care Med 2008;36:145-50. [Crossref] [PubMed]
- Kishimoto S, Uwatoko S, Ito K, et al. Normal serum concentration of IgG subclasses in the Japanese adults. Jpn J Clin Immun 1994;17:535-45. [Crossref]
- Dietz S, Lautenschläger C, Müller-Werdan U, et al. Serum IgG levels and mortality in patients with severe sepsis and septic shock: The SBITS data. Med Klin Intensivmed Notfmed 2017;112:462-70. [Crossref] [PubMed]


