
Cardiac electrical storm induced by anesthesia was successfully managed during surgery: a case report
Introduction
Cardiac electrical storm, also known as ventricular tachycardia (VT) storm, is defined as 3 or more episodes of VT or ventricular fibrillation (VF) within a 24-hour period (1). Clinical causes of cardiac electrical storm are unknown in approximately 66% of cases; in the remaining cases, the etiology has been attributed to decompensated heart failure, acute myocardial ischemia, or metabolic disturbance. Disturbed K+ metabolism accounts for the vast majority of electrolyte-induced arrhythmias (2).
General anesthesia (GA) has been associated with hemodilution and increased plasma volume. For example, Damén et al. (3). studied cardiac surgery patients who received no intravenous fluids during the first 70 minutes after anesthesia induction. By 10 minutes post-induction, a 10–15% increase in plasma volume was detected, which persisted throughout the 70-minute observation interval. This was accompanied by a decreased hematocrit. Electrolytes were not measured, but this could provide a plausible mechanism for onset of acute hypokalemia.
VF is more common in patients with structural heart disease, such as coronary heart disease, cardiomyopathy, myocarditis and other structural heart disease, especially when complicated with cardiac insufficiency. It can also be seen in severe ischemia, hypoxia, and pre-excitation with atrial fibrillation, electric shock, digitalis poisoning, application of antiarrhythmic drugs, acid-base balance disorders, water and electrolyte disorders, etc. However, the cause of VF in GA patients without heart disease is not clear, so GA-induced VF is rare and there is no specific treatment plan, so the treatment is challenging.
We report a case of cardiac storm in a patient undergoing superficial surgery under GA and discuss potential etiologies for this previously unreported adverse response to anesthesia and surgery. This case is unique in that the patient was selected for a cardiac storm during superficial surgery under GA to analyze the potential etiology of anesthetic and surgical adverse reactions, which had not been previously reported. This case report will help guide future clinical practice and study design. We present the following article in accordance with the CARE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-498/rc).
Case presentation
A 57-year-old Chinese man was scheduled for resection of lip neoplasms and local skin flap transplantation under GA on 25 February, 2016. He presented with clinical symptoms such as exudation, bad breath, restricted mouth opening, and mucous leukoplakia on local skin. He had a history of gastrectomy due to gastric cancer 20 years earlier. The preoperative review of systems, physical examination, and laboratory investigations revealed no evidence of heart disease. There were no abnormal findings in preoperative blood chemistry performed 5 days before surgery. His hematocrit was 51% and serum potassium was 4.4 mmol/L. The patient weighed 77 kg, with a height of 174 cm. After biopsy, the patient was diagnosed with a stage II lip tumor. The whole event is shown in (Figure 1). All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
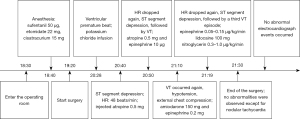
Case treatment
The patient underwent surgical treatment and was admitted to the operating room at 6:30 pm. The electrocardiogram (ECG), pulse oximeter, and non-invasive blood pressure (NIBP) were monitored routinely. Before anesthesia induction, midazolam 2 mg was given intravenously for sedation. At 6:40 pm, anesthesia was induced with intravenous sufentanil 50 µg, etomidate 22 mg, and cisatracurium 15 mg. The trachea was intubated. Sevoflurane and remifentanil were used for GA maintenance. The patient was stable during induction, without any significant hypotension. Surgery began at 7:20 pm. His NIBP increased from 120/80 to 140/90 mmHg, and heart rate (HR) from 70 to 90 beats per minute. In response, 20 µg dexmedetomidine was given intravenously.
The procedure was uneventful until 8:28 pm, when several ventricular premature beats were first monitored; prior to then, there had been no abnormalities in oxygen saturation (SpO2) or end-tidal CO2. A total of 900 mL intravenous fluid, including hydroxyethyl starch (HES) 200/0.5 (500 mL), Ringer’s solution (300 mL), and normal saline (100 mL) had been given up to this point. No other medications had been given. The left radial artery was cannulated immediately for arterial blood pressure (BP) measurement and blood gas analysis. The hematocrit was 30% and potassium was 2.7 mmol/L. An intravenous potassium chloride infusion was initiated at the rate of 1 g/hour.
At 8:40 pm, the ECG showed ST segment depression, and HR was 48 beats per minute. Atropine 0.5 mg was administered intravenously. The HR increased to 80 beats per minute and the ST segment returned to baseline.
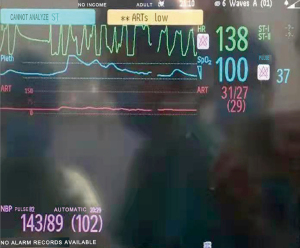
At 8:50 pm, the HR dropped again with significant ST segment depression, followed by VT (Figure 2). Atropine 0.5 mg and epinephrine 10 µg were given intravenously. Both BP and HR improved, but ST segment depression persisted. The VT returned to sinus rhythm. Blood gas analysis measured potassium at 4.7 mmol/L, with other values being normal.

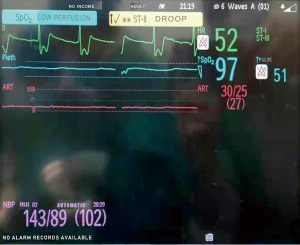
At 9:10 pm, VT occurred again. It was polymorphic accompanied with profound hypotension (Figure 3). The surgeons were asked to interrupt the procedure and chest compressions were initiated immediately. Amiodarone 150 mg and epinephrine 0.2 mg were given intravenously. The BP recovered and VT resolved. Chest compressions were stopped. The surgeons resumed the procedure.
At 9:19 pm, the HR dropped again accompanied by ST segment depression (Figure 4), followed by a third VT episode (a screen shot was not captured). Epinephrine 0.05–0.15 µg/kg/min and lidocaine 100 mg were given intravenously. Coronary artery spasm was considered in the differential diagnosis and intravenous nitroglycerin 0.3–1.0 µg/kg/min was begun. Without requirement for defibrillation, the patient’s heart rhythm and BP returned to baseline within 30 minutes. The operation was completed at 9:30 pm. A 12-lead ECG was recorded emergently, showing nothing abnormal except for nodal tachycardia.
Post operation
The patient was transferred to the intensive care unit (ICU) after the operation. The trachea remained intubated. He was hemodynamically stable. Blood tests showed no abnormalities other than serum cardiac troponin I values of 0.81 and 4.32 ng/mL at 1:11 am and 6:00 am on 26 February, respectively. The trachea was extubated at 10:00 am on 26 February. The patient was discharged from the ICU to a monitored ward on 27 February. During this interval, no abnormal electrocardiographic events occurred. The patient was discharged from the hospital on 20 March after one course of chemotherapy. Over the ensuing 6 months, several follow-ups were conducted, and the patient reported no recurrent cardiac events. During the treatment and follow-up period, the patient actively cooperated and had good compliance.
Discussion
Cardiac electrical storm, also known as electrical storm, is a syndrome characterized by recurrent VF of tachycardia. It is a major clinical challenge and is often unresponsive to conventional drug therapy (4).Little data exists regarding the manifestation of cardiac electrical storm during GA, especially in patients without cardiac disease, which is in part due to the extremely low incidence of this disorder. The cause of the witnessed cardiac storm in our patient is unclear.
Anesthesia induction with commonly used anesthetics often causes systemic hypotension by direct vasodilation, sympathetic inhibition, and myocardial depression (5). Substantive systemic hypotension did not precede the ventricular arrhythmias and the absence of any history of cardiac disease in our patient make cardiac ischemia an unlikely primary etiology.
Anesthesia induction has been associated with hemodilution and increased plasma volume (6). For our patient, the hematocrit decreased from a preoperative value of 51% to 30% measured 115 minutes after anesthesia induction. The infusion of 900 mL of intravenous fluid to this 77 kg patient is insufficient to explain this 41.2% decrease in hematocrit. Estimated blood loss was negligible. Thus, a different mechanism must have induced the decreased hematocrit and associated hypokalemia.
Acute hemodilution, caused by an increase in the circulating plasma volume due to autotransfusion, is a previously reported phenomena (3,6). Sano et al. (6) documented this in patients undergoing minor surgery and hypothesized that this was attributable to decreased arterial and capillary pressures associated with anesthesia induction allowing ultrafiltration into the intravascular department and hemodilution. Notably, the increased circulating blood volume persisted through 90 minutes post-induction observation, but normalized during recovery from anesthesia. Damén et al. (3). showed these changes in blood volume could be avoided by using a norepinephrine infusion sufficient to maintain BP at pre-induction levels. In our case, to maintain the normal BP, fluids were given during induction and maintenance of anesthesia, which could have further decreased the hematocrit. Hypokalemia is one of consequences of hemodilution. It could induce ventricular arrhythmia, and did so, in this case.
Bradycardia, ST segment depression, and hypokalemia were the first clinical signs of the untoward circumstance of our patient. Early ECG signs of hypokalemia were not evident (7), although these are often absent at the potassium concentration measured in our patient. New onset hypokalemia has been associated with an increased QT interval. This may in turn interact with various anesthetic medications or adrenergic stress to further prolong the QT interval and predispose the patient to cardiac arrhythmias (8,9). We can only speculate that this mechanism accounts for the cardiac electrical storm witnessed in our patient.
Amiodarone and β-blockade have been recommended for treatment of electrical storm under the hypothesis that electrical storm is caused by sympathetic overdrive (10,11). There is little data available to direct management of intraoperative electrical storm (12). At the first occurrence of bradycardia, we did not recognize the likely etiology and evolution of electrical storm. We administered atropine, which resolved bradycardia. However, this was followed 10 minutes later by VT, which we treated with atropine and epinephrine. Epinephrine and amiodarone were given in the second episode; epinephrine and lidocaine were used to treat the third. While each event resolved in response to therapy, it is plausible we contributed to the cascade of arrhythmias by administering epinephrine.
To the authors’ knowledge, this is the first case report of a cardiac electrical storm occurring in patient without cardiac disease during GA. More information is required to understand the mechanisms of cardiac electrical storms in the context of anesthesia, especially in patients with no preoperative evidence of cardiac pathology.
The patient said: “I have never had any heart disease before, and no one expected that there would be cardiac electrical storm during the operation, which may be related to the change of some indicators during the operation, but fortunately I got timely treatment from the doctor”.
Conclusions
Abrupt onset hypokalemia and hemodilution occurring with anesthesia induction were temporally associated with new onset recurrent ventricular arrhythmia during GA. Autotransfusion is proposed to be the mechanism for hemodilution after anesthesia induction. For this kind of case needs as soon as possible electric defibrillation and electric cardioversion, timely intravenous application effective anti-arrhythmic drugs and other treatment measures.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-498/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-498/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Elsokkari I, Sapp JL. Electrical storm: Prognosis and management. Prog Cardiovasc Dis 2021;66:70-9. [Crossref] [PubMed]
- Laslett DB, Cooper JM, Greenberg RM, et al. Electrolyte Abnormalities in Patients Presenting With Ventricular Arrhythmia (from the LYTE-VT Study). Am J Cardiol 2020;129:36-41. [Crossref] [PubMed]
- Damén T, Reinsfelt B, Redfors B, et al. Pressure-dependent changes in haematocrit and plasma volume during anaesthesia, a randomised clinical trial. Acta Anaesthesiol Scand 2016;60:560-8. [Crossref] [PubMed]
- Hulata DF, Le-Wendling L, Boezaart AP, et al. Stellate ganglion local anesthetic blockade and neurolysis for the treatment of refractory ventricular fibrillation. A A Case Rep 2015;4:49-51. [Crossref] [PubMed]
- Shah SB, Chowdhury I, Bhargava AK, et al. Comparison of hemodynamic effects of intravenous etomidate versus propofol during induction and intubation using entropy guided hypnosis levels. J Anaesthesiol Clin Pharmacol 2015;31:180-5. [Crossref] [PubMed]
- Sano Y, Sakamoto A, Oi Y, et al. Anaesthesia and circulating blood volume. Eur J Anaesthesiol 2005;22:258-62. [Crossref] [PubMed]
- Diercks DB, Shumaik GM, Harrigan RA, et al. Electrocardiographic manifestations: electrolyte abnormalities. J Emerg Med 2004;27:153-60. [Crossref] [PubMed]
- Owczuk R, Wujtewicz MA, Zienciuk-Krajka A, et al. The influence of anesthesia on cardiac repolarization. Minerva Anestesiol 2012;78:483-95. [PubMed]
- Castelletti S, Winkel BG, Schwartz PJ. Remote Monitoring of the QT Interval and Emerging Indications for Arrhythmia Prevention. Card Electrophysiol Clin 2021;13:523-30. [Crossref] [PubMed]
- Margos PN, Margos NP, Delakis IK, et al. Digitalis-related electric storm short after ICD implantation. Hellenic J Cardiol 2021;62:480-4. [Crossref] [PubMed]
- Elsokkari I, Tsuji Y, Sapp JL, et al. Recent Insights Into Mechanisms and Clinical Approaches to Electrical Storm. Can J Cardiol 2022;38:439-53. [Crossref] [PubMed]
- Guarracini F, Casella M, Muser D, et al. Clinical management of electrical storm: a current overview. J Cardiovasc Med (Hagerstown) 2021;22:669-79. [Crossref] [PubMed]
(English Language Editor: J. Jones)


