
Efficacy and safety of sacubitril/valsartan in the treatment of middle-aged and elderly patients with hypertension: a systematic review and meta-analysis of randomized controlled trials
Introduction
Hypertension is a common cardiovascular disease and a well-known risk factor for other cardiovascular diseases. According to the American College of Cardiology/American Heart Association (ACC/AHA) High Blood Pressure Guideline released in 2017, nearly 46% of Americans have hypertension (1). A study showed that the global number of hypertensive patients will increase to 1.5 billion by 2025 (2). In China, a survey showed that the overall prevalence of hypertension among the elderly is 53.2%, affecting 51.1% of males and 55.3% of females (3). With the development of new antihypertensive drugs and the launch of large-scale antihypertensive trials, the drug treatment of hypertension has been rapidly developed, and some trials have shown that antihypertensive drugs have great benefits in reducing the risk of major cardiovascular events and all-cause mortality (4). At present, the first-line antihypertensive drugs recommended by international hypertension guidelines mainly include calcium channel blockers (CCB), angiotensin converting enzyme inhibitors (ACEI), angiotensin II receptor blockers (ARBs), diuretics, and β blockers (5,6). However, whether within China, the United States, or the broader global population, the majority of middle-aged and elderly hypertensive patients still have poor blood pressure control (5,7,8). Therefore, it is necessary to keep looking for better antihypertensive drugs to help patients achieve the target blood pressure and reduce the incidence of cardiovascular events.
Sacubitril/valsartan, an angiotensin receptor-neprilysin inhibitor (ARNI), has attracted great interest following its approval for the treatment of heart failure with reduced ejection fraction (HFrEF). A prospective clinical study comparing sacubitril/valsartan with enalapril to determine the effects of sacubitril/valsartan in patients with HFrEF showed that these patients not only had lower rates of mortality and hospitalization for heart failure, but also had a lower systolic blood pressure by 3.2 mmHg (9). Some studies have also shown that sacubitril/valsartan has better antihypertensive effect than ACEI and ARBs in heart failure patients with hypertension (10,11). In addition, a study showed that sacubitril/valsartan reduced the incidence of renal impairment and hyperkalemia compared with traditional antihypertensive drugs (12). In recent years, ethics committees have approved several randomized controlled trials (RCTs) to evaluate the efficacy and safety of sacubitril/valsartan as an antihypertensive drug, mostly for the treatment of hypertension in middle-aged and elderly patients. In these RCTs, results from one study to another were not completely consistent. In terms of efficacy, compared with other antihypertensive drugs, one study (13) suggested that sacubitril/valsartan had a better antihypertensive effect, while the other study (14) found that the efficacy of sacubitril/valsartan was not much different. Similarly, in terms of safety, compared with other antihypertensive drugs, one study (15) considered sacubitril/valsartan to be safer, one study (16) suggested that sacubitril/valsartan had no significant difference in safety, and another study (13) found that sacubitril/valsartan was more prone to adverse events (AEs). Based on these inconsistent results, a meta-analysis is suitable to investigate the efficacy and safety of sacubitril/valsartan in the treatment of middle-aged and elderly hypertensive patients. In order to determine and quantify the efficacy and safety of sacubitril/valsartan in the treatment of middle-aged and elderly patients with hypertension, we evaluated the impact of different antihypertensive drugs on outcomes in middle-aged and elderly hypertensive patients by performing a systematic review and meta-analysis of RCTs between sacubitril/valsartan and other antihypertensive drugs. We summarized and analyzed the clinical trial data, hoping to provide a reliable basis for the clinical application of sacubitril/valsartan in the treatment of hypertension. We present the following article in accordance with the PRISMA reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-503/rc) (17).
Methods
Evidence acquisition
A prospective protocol of objectives, literature search strategies, inclusion and exclusion criteria, outcome measurements, and methods of statistical analysis was prepared in advance.
Literature-search strategy
A literature search was conducted in February 2022 and was not restricted by region, publication type, or language. The main data sources were the electronic databases of PubMed, Embase, and Web of Science. We searched [Title/Abstract] for the following terms and their combinations: sacubitril/valsartan OR Entresto OR LCZ696 OR AHU377 AND hypertension OR high blood pressure. Related article functions were also used to extend the search, and computer searches were complemented by manual searches of all bibliography that retrieved research, review articles, and conference abstracts. When multiple reports describing the same population are published, the latest or complete report was used.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (I) type: published RCTs comparing sacubitril/valsartan with other antihypertensive drugs in middle-aged and elderly patients with hypertension; (II) participants: middle-aged and elderly patients aged ≥55 years with clinically diagnosed hypertension; (III) interventions: the experimental group was only treated with sacubitril/valsartan, and the control group was treated with one of the other antihypertensive drugs (such as CCB, ARBs, β blockers, etc.); (IV) outcomes: (i) mean reductions in sitting systolic blood pressure (msSBP); (ii) mean reductions in sitting diastolic blood pressure (msDBP); (iii) 24-hour mean reductions in ambulatory systolic blood pressure (24-h maSBP); (iv) 24-hour mean reductions in ambulatory diastolic blood pressure (24-h maDBP); (v) AEs; (vi) serious adverse events (SAE); (vii) discontinuations due to AEs.
The exclusion criteria were as follows: (I) patients aged <55 years; (II) editorials; (III) letters to editor; (IV) review articles; (V) case reports; (VI) unavailable full texts; (VII) meeting abstracts; (VIII) abstract data not extractable; and (IX) animal experimental studies.
Data extraction and outcomes of interest
The data in the included studies were independently extracted and summarized by three authors (HX Wu, KK Liu, and BN Li). Any disagreement was resolved by the adjudicating senior authors (HX Wu and JC Jin).
According to the purpose, the name of the first author, the country/region of origin, the time of publication, the design of study, the plan and timing of intervention, the total number of middle-aged and elderly hypertensive patients, the number of patients in experimental group and control group, the average age, the average body mass index (BMI), the baseline blood pressure, and the outcomes were extracted from each included study.
The outcomes used to evaluate the efficacy of sacubitril/valsartan in the treatment of middle-aged and elderly hypertensive patients were msSBP, msDBP, 24-h maSBP, and 24-h maDBP, while outcomes used to evaluate the safety of sacubitril/valsartan were AEs, SAE, and discontinuations due to AEs.
Quality assessment and statistical analysis
The Cochrane Collaboration’s tool for assessing risk of bias (The Nordic Cochrane Centre, Copenhagen, Denmark) was used for all included RCTs, including: (I) random sequence generation (selection bias); (II) allocation concealment (selection bias); (III) blinding of participants and personnel (performance bias); (IV) blinding of outcome assessment (detection bias); (V) incomplete outcome data (attrition bias); (VI) selective reporting (reporting bias); and (VII) other bias.
All meta-analyses were performed using Review Manager 5.3 (RevMan 5.3; Cochrane Collaboration, Oxford, UK). Continuous variables such as msSBP, msDBP, 24-h maSBP, and 24-h maDBP were represented by mean difference (MD) and its 95% confidence interval (CI), and binary variables such as AEs, SAE, and discontinuations due to AEs were represented by odds ratio (OR) and its 95% CI. The chi-square test was used to analyze the heterogeneity of the included studies, with significance set at P<0.10. If P>0.10 and I2≤50%, indicating the homogeneity between studies was good, and the fixed-effects model was used. If P≤0.10 and/or I2>50%, indicating that there was heterogeneity among studies, and the random-effects model was used (18).
Sensitivity analysis was performed for outcomes with greater heterogeneity, and individual studies were excluded in turn to identify potential causes. Funnel plots were used to screen for potential publication bias.
Results
Evidence synthesis
In all, seven studies including 3,323 cases (1,899 cases of sacubitril/valsartan and 1,424 cases of other antihypertensive drugs) fulfilled the predetermined inclusion criteria, and were eventually included in the analysis (13-16,19-21). All seven publications were full-text articles. An examination of the references listed in these publications and review articles of these studies did not result in any further research evaluations. The consistency between the two reviewers (HX Wu and JC Jin) was 97% on study selection and 94% on trial quality assessment.
Characteristics of studies
Based on the literature search strategy, 355 articles were identified as potentially relevant. After a preliminary review of 355 articles by reading titles and abstracts, 322 articles were excluded and 33 articles were retrieved, which were further reviewed in full text. A total of 26 of the studies were excluded on the basis of selection criteria, leaving seven studies to be evaluated. The flow chart of the study selection process and the reasons for excluding the studies are shown in Figure 1.
Tables 1,2 show the characteristics and data of the included studies. Seven studies (13-16,19-21) included in this meta-analysis were published between 2017 and 2019, which came from America (15,20), Britain (14), Germany (21), Mainland China (16), Taiwan China (19), and Thailand (13).
Table 1
| Included study | Country/region | Year | Blind | RCT | Duration (weeks) | Intervention plan (mg/d) | Number of patients | Design of study | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Experimental group | Control group | Total | Experimental group | Control group | ||||||||
| Izzo et al. (20) | America | 2017 | DB | Yes | 8 | Sacubitril/valsartan 400 | Valsartan 320 | 285 | 142 | 143 | Multicenter, parallel-group study | |
| Schmieder et al. (21) | Germany | 2017 | DB | Yes | 52 | Sacubitril/valsartan 400 | Olmesartan 40 | 114 | 57 | 57 | Multicenter, double-blind, parallel-group study | |
| Supasyndh et al. (13) | Thailand | 2017 | DB | Yes | 14 | Sacubitril/valsartan 200, 400 | Olmesartan 20, 40 | 588 | 296 | 292 | Multicenter, parallel-group study | |
| Wang et al. (19) | Taiwan, China | 2017 | DB | Yes | 4 | Sacubitril/valsartan 400 | Valsartan 320 | 72 | 36 | 36 | Multicenter, crossover study | |
| Williams et al. (14) | Britain | 2017 | DB | Yes | 52 | Sacubitril/valsartan 200 | Olmesartan 20 | 454 | 229 | 225 | Multicenter, parallel-group study | |
| Cheung et al. (15) | America | 2018 | DB | Yes | 8 | Sacubitril/valsartan 200 | Olmesartan 20 | 375 | 188 | 187 | Multicenter, double-blind, parallel-group study | |
| Huo et al. (16) | Mainland China | 2019 | DB | Yes | 8 | Sacubitril/valsartan 200, 400 | Olmesartan 20 | 1,435 | 200 mg 479; 400 mg 472 | 484 | Multicenter, parallel-group study | |
DB, double blind; RCT, randomized controlled trial.
Table 2
| Included study | Group | Age (years) | BMI (kg/m2) | Baseline SBP (mmHg) | Baseline DBP (mmHg) | Outcomes |
|---|---|---|---|---|---|---|
| Izzo et al. (20) | Experimental | 61.2±10.6 | 29.3±5.5 | 159.6±7.0 | 90.9±8.9 | (I), (II), (III), (IV), (V), (VI) |
| Control | 62.0±11.5 | 30.0±5.3 | 160.0±7.3 | 90.2±9.4 | ||
| Schmieder et al. (21) | Experimental | 60.5±7.8 | 28.1±4.5 | 155.3±9.0 | 92.7±8.8 | (I), (II) |
| Control | 59.2±13.1 | 28.6±3.9 | 155.0±9.1 | 91.7±8.7 | ||
| Supasyndh et al. (13) | Experimental | 70.5±4.67 | 24.3±3.15 | 160.5±8.41 | 84.6±9.74 | (I), (II), (III), (IV), (V), (VI) |
| Control | 70.9±4.67 | 24.6±3.24 | 160.0±7.99 | 85.2±9.83 | ||
| Wang et al. (19) | Experimental | 55.7±12.5 | 26.4±3.8 | 147.0±9.7 | 90.2±6.9 | (I), (II), (III), (IV), (V) |
| Control | 58.9±7.5 | 25.7±2.9 | 147.5±12.1 | 90.4±7.2 | ||
| Williams et al. (14) | Experimental | 68.2±5.73 | 28.6±4.47 | 158.4±13.41 | 87.8±9.72 | (I), (II), (III), (IV), (V), (VI), (VII) |
| Control | 67.2±5.97 | 29.1±4.90 | 158.8±13.48 | 89.9±10.38 | ||
| Cheung et al. (15) | Experimental | 57.1±10.19 | 30.5±5.86 | 157.1±9.54 | 90.4±10.24 | (I), (II), (III), (IV), (V), (VI), (VII) |
| Control | 58.0±9.09 | 30.6±5.09 | 157.8±10.17 | 91.2±8.89 | ||
| Huo et al. (16) | Experimental (200 mg) | 57.5±10.17 | 26.4±3.91 | 158.0±7.15 | 90.7±9.37 | (I), (II), (III), (IV), (V), (VI), (VII) |
| Experimental (400 mg) | 58.1±9.71 | 26.3±3.56 | 157.9±6.73 | 89.8±9.46 | ||
| Control | 57.4±10.14 | 26.4±3.92 | 158.0±6.53 | 90.8±9.57 |
Outcomes: (I) mean reductions in sitting systolic blood pressure (msSBP); (II) mean reductions in sitting diastolic blood pressure (msDBP); (III) 24-hour mean reductions in ambulatory systolic blood pressure (24-h maSBP); (IV) 24-hour mean reductions in ambulatory diastolic blood pressure (24-h maDBP); (V) adverse events (AEs); (VI) serious adverse events (SAE); (VII) discontinuations due to AEs. BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure.
The studies included in this meta-analysis were all RCTs. A total of 3,323 middle-aged and elderly hypertensive patients were included, of whom 1,899 were treated with sacubitril/valsartan and 1,424 with valsartan or olmesartan. Three studies had an 8-week intervention (15,16,20), and the remaining studies had an intervention duration of 4 weeks (19), 14 weeks (13), and 52 weeks (14,21). In the experimental group, sacubitril/valsartan was administered at doses of 200 and 400 mg/d in two studies (13,16), 200 mg/d in two studies (14,15), and 400 mg/d in three studies (19-21). The antihypertensive drugs used in the control group included valsartan (19,20) and olmesartan (13-16,21), of which valsartan was 320 mg/d and olmesartan was 20 and 40 mg/d.
Methodological quality of included studies
The quality of included studies was generally high. All included studies described the random methods, blinded participants, investigators, and outcome evaluators, and detailed inclusion and exclusion criteria. One study (20) did not clearly describe the allocation concealment, and three studies (13,15,21) were lost to follow-up and did not describe the cause of loss. After reading all the included studies, no other bias was found. The risk of bias graph and risk of bias summary of the included studies are shown in Figure 2.
Outcomes to evaluate efficacy
msSBP and msDBP
A total of 7 studies (13-16,19-21) reported the effects of sacubitril/valsartan on msSBP and msDBP. Data were extracted and combined for analysis, and the analysis results are shown in Figure 3. In the analysis with msSBP as the outcome, P=0.005 and I2=64%, indicating that there was heterogeneity among studies, so the random-effects model was used for analysis. In contrast, because the heterogeneity between studies was acceptable, the fixed-effects model was used for the analysis of msDBP. The results showed that the effects of reducing msSBP and msDBP in the experimental group were significantly better than that in the control group (msSBP: MD =−4.70, 95% CI: −5.79 to −3.61, P<0.001; msDBP: MD =−2.29, 95% CI: −2.53 to −2.04, P<0.001).
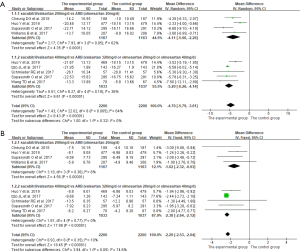
24-h maSBP and 24-h maDBP
A total of 6 studies (13-16,19,20) reported 24-h maSBP and 24-h maDBP as the outcomes. As shown in Figure 4, P<0.10 and I2>50% indicated that there was heterogeneity among studies, so the random-effects model was used for analysis. The results of meta-analysis showed that the effects of reducing 24-h maSBP and 24-h maDBP in the experimental group was significantly better than that in the control group (24-h maSBP: MD =−3.36, 95% CI: −4.08 to −2.64, P<0.001; 24-h maDBP: MD =−1.49, 95% CI: −1.99 to −0.99, P<0.001).
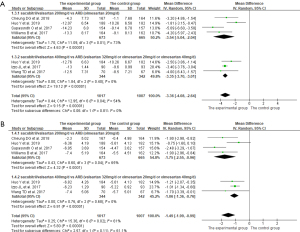
Outcomes to evaluate safety
The AEs, SAE, and discontinuations due to AEs were reported in six (13-16,19,20), five (13-16,20), and three (14-16) studies, respectively. In the analysis with these three outcomes, there was good homogeneity among the studies, so the fixed-effects model was adopted for analysis. The analysis results showed that there was no significant difference in the incidence of AEs, SAE, and discontinuations due to AEs between the experimental group and the control group (AEs: OR =1.14, 95% CI: 1.00 to 1.31, P=0.06; SAE: OR =1.06, 95% CI: 0.64 to 1.76, P=0.81; discontinuations due to AEs: OR =0.86, 95% CI: 0.51 to 1.46, P=0.58). The results are shown in Figure 5.
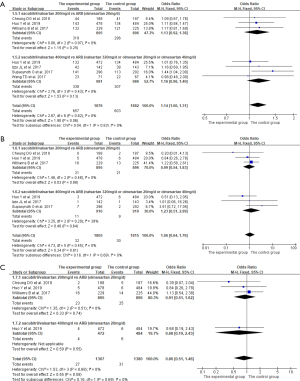
Subgroup analysis
Subgroup analysis was performed on different doses of sacubitril/valsartan. As shown in Figures 3-5 and Table 3, no matter whether the dose of sacubitril/valsartan was 200 or 400 mg/d in the experimental group, the effects of reducing msSBP, msDBP, 24-h maSBP, and 24-h maDBP were superior to that in the control group (all P<0.01), and the incidence of AEs, SAE, and discontinuations due to AEs had no significant difference compared with the control group (all P>0.05).
Table 3
| Outcomes | Doses (mg/d) | Number of included studies | Number of patients | MD/OR | 95% CI | P value |
|---|---|---|---|---|---|---|
| msSBP | 200 | 4 | 1,167 | −4.11 | −5.96 to −2.26 | <0.01 |
| 400 | 5 | 1,033 | −5.20 | −6.26 to −4.14 | <0.01 | |
| msDBP | 200 | 4 | 1,167 | −1.62 | −2.32 to −0.93 | <0.01 |
| 400 | 5 | 1,033 | −2.38 | −2.64 to −2.12 | <0.01 | |
| 24-h maSBP | 200 | 4 | 673 | −3.54 | −5.04 to −2.04 | <0.01 |
| 400 | 3 | 344 | −3.35 | −3.70 to −3.01 | <0.01 | |
| 24-h maDBP | 200 | 4 | 673 | −1.75 | −2.55, −0.96 | <0.01 |
| 400 | 3 | 344 | −1.06 | −1.36 to −0.76 | <0.01 | |
| AEs | 200 | 3 | 895 | 1.13 | 0.92 to 1.38 | 0.25 |
| 400 | 4 | 981 | 1.16 | 0.96 to 1.40 | 0.13 | |
| SAE | 200 | 3 | 895 | 0.99 | 0.54 to 1.83 | 0.98 |
| 400 | 3 | 910 | 1.23 | 0.51 to 2.99 | 0.64 | |
| Discontinuations due to AEs | 200 | 3 | 895 | 0.91 | 0.51 to 1.62 | 0.74 |
| 400 | 1 | 472 | 0.68 | 0.19 to 2.43 | 0.55 |
msSBP, mean reductions in sitting systolic blood pressure; msDBP, mean reductions in sitting diastolic blood pressure; 24-h maSBP, 24-hour mean reductions in ambulatory systolic blood pressure; 24-h maDBP, 24-hour mean reductions in ambulatory diastolic blood pressure; AEs, adverse events; SAE, serious adverse events; MD, mean difference; OR, odds ratio; CI, confidence interval.
Sensitivity analysis and publication bias
Meta-analysis showed that significant heterogeneity appeared in msSBP, 24-h maSBP, and 24-h maDBP in the experimental group compared with the control group (msSBP: P=0.005, I2=64%; 24-h maSBP: P=0.04, I2=54%; 24-h maDBP: P=0.02, I2=61%). Therefore, the included studies were eliminated one by one, and then the remaining studies were combined for sensitivity analysis. The results are shown in Figure 6. Sensitivity analysis found that heterogeneity was significantly reduced after eliminating the studies of Huo et al. (16) and Izzo et al. (20) (msSBP: P=0.55, I2=0%; 24-h maSBP: P=0.43, I2=0%; 24-h maDBP: P=0.72, I2=0%), but the effects of the experimental group in reducing msSBP, 24-h maSBP, and 24-h maDBP were still significantly better than that of the control group (msSBP: MD =−5.28, 95% CI: −6.44 to −4.13, P<0.001; 24-h maSBP: MD =−4.20, 95% CI: −5.08 to −3.32, P<0.001; 24-h maDBP: MD =−2.12, 95% CI: −2.65 to −1.60, P<0.001).

Since the number of included studies in this meta-analysis was less than 10, the test power of funnel plot to evaluate potential publication bias was low, so publication bias analysis was not carried out in this meta-analysis.
Discussion
Currently, theories suggest that hyperactivity of the sympathetic nervous system (SNS), excessive activation of renin-angiotensin-aldosterone system (RAAS), and the involvement of natriuretic peptides (NPs) system are the main pathophysiological mechanisms leading to essential hypertension. For middle-aged and elderly patients with hypertension, the sodium excretion capacity of the kidneys gradually weakens with age, resulting in water and sodium retention, causing a compensatory increase in blood pressure; vascular remodeling results in stenosis of lumen and physiological enhancement of platelet function, resulting in increased peripheral vascular resistance, which are also important factors that cannot be ignored (22). Up to now, hypertension remains the leading cause of morbidity and mortality from cardiovascular diseases and is an important risk factor for most cardiovascular diseases, such as stroke, hypertensive heart disease, myocardial infarction, and heart failure (23,24). A trial led by the Systolic Blood Pressure Intervention Trial (SPRINT) research team showed that the prevalence of hypertension in Americans has risen from 31.9% to 46%, with more than 50% needing better control of high blood pressure (25). In China, there were also relevant statistics showing that 2.54 million people died of hypertension in 2017 alone (26). Therefore, it is of great significance for patients with hypertension, especially middle-aged and elderly patients, to treat hypertension with medication and achieve blood pressure targets to reduce the occurrence of coronary heart disease and heart failure.
Sacubitril/valsartan is an ARNI. It is a compound composed of sacubitril and valsartan in the ratio of 1 mol:1 mol, which can act on NPs and RAAS simultaneously to exert an antihypertensive effect. Sacubitril, the precursor of neprilysin inhibitor, can be hydrolyzed into the neprilysin inhibitor LBQ657 by carboxylesterase in the liver. By inhibiting the activity of neprilysin, LBQ657 can increase the content of NPs in the body, promote natriuresis, diuresis, and vasodilation, so as to achieve the purpose of lowering blood pressure (27,28). Another component, valsartan, as an ARB, can selectively block and inhibit the type 1 receptor (AT1) of angiotensin II, thereby inhibiting RAAS to reduce the release of aldosterone, and regulating the reabsorption of sodium and potassium by the kidneys to reduce water and sodium retention, thus achieving the purpose of lowering blood pressure (29). In addition, the synergistic effect of sacubitril and valsartan can also prevent myocardial fibrosis and hypertrophy, inhibit renal fibrosis, increase glomerular filtration rate, and inhibit atherosclerosis, which is better than the currently commonly used antihypertensive drugs.
In this meta-analysis, we systematically analyzed the efficacy and safety of sacubitril/valsartan compared with other antihypertensive drugs in the treatment of hypertension in middle-aged and elderly patients. According to the results of meta-analysis, in terms of efficacy, sacubitril/valsartan can reduce msSBP, msDBP, 24-h maSBP, and 24-h maDBP better than other antihypertensive drugs, that is, sacubitril/valsartan has a better antihypertensive efficacy. In terms of safety, compared with other antihypertensive drugs, sacubitril/valsartan has no significant difference in the incidence of AEs, SAE, and discontinuations due to AEs, that is, the safety of sacubitril/valsartan is basically the same as other antihypertensive drugs, especially ARBs. In addition, the results of subgroup analysis showed that in terms of efficacy, the antihypertensive effect of sacubitril/valsartan was superior to that of other antihypertensive drugs either at 200 or 400 mg/d. In terms of safety, whether sacubitril/valsartan was dosed at 200 or 400 mg/d, compared with other antihypertensive drugs, there was still no significant difference in the incidence of AEs, SAE, and discontinuations due to AEs. In general, sacubitril/valsartan has superior antihypertensive efficacy compared with other antihypertensive drugs such as olmesartan and valsartan, and its safety is basically the same.
During the meta-analysis, we found significant heterogeneity in msSBP, 24-h maSBP, and 24-h maDBP between the experimental group and the control group, so sensitivity analysis was performed. We found that heterogeneity was significantly reduced after eliminating the studies by Huo et al. (16) and Izzo et al. (20), which may be due to the following reasons. In the study of Izzo et al. (20), they only included patients with isolated systolic hypertension (ISH), whose blood pressure was characterized by systolic blood pressure ≥140 mmHg and diastolic blood pressure <90 mmHg. However, other included studies were not limited to ISH patients, which may have resulted in heterogeneity. The study of Huo et al. (16) was only carried out in Asia, and hypertension in Asians has some special characteristics such as salt sensitivity (19). However, most of the other included studies did not strictly limit the race and scope of the participants, so ethnic factors may have led to heterogeneity. After eliminating these two studies, the results showed that the antihypertensive effect of sacubitril/valsartan was still better, which further verified the reliability of the results of this meta-analysis.
The results of this meta-analysis are consistent with those of Zhao et al. (30) and De Vecchis et al. (31). By comparing our study with the two studies mentioned above, we found some advantages of our meta-analysis. In Zhao et al.’s study (30), although it was concluded that the antihypertensive effect of sacubitril/valsartan was significantly better than that of ARBs, there was significant heterogeneity in the results of their meta-analysis, with I2 as high as 100% in the analysis of msSBP, maSBP, and maDBP as outcomes. Moreover, sensitivity analysis was not conducted in the case of large heterogeneity in their study, which greatly reduced the credibility of the results. For De Vecchis et al. (31), although the theme of their study was the efficacy and safety of sacubitril/valsartan on hypertension in the elderly, our study was superior to theirs in that it included more studies, more outcome indicators, and stronger timeliness. Therefore, this meta-analysis is more novel, and the results are more reliable.
The results of this meta-analysis showed that sacubitril/valsartan has superior efficacy in lowering blood pressure, and its safety is basically equivalent to that of olmesartan, valsartan, and other antihypertensive drugs, suggesting that sacubitril/valsartan can bring greater benefits to middle-aged and elderly patients with hypertension. Although it has not yet been listed as a first-line antihypertensive drug for the treatment of hypertension, it has a very promising prospect and can be used as a new choice for hypertension treatment, especially for the treatment of hypertension in middle-aged and elderly patients (14). However, it is worth noting that some studies have found that although the safety of sacubitril/valsartan is basically similar to that of ARBs, it may increase the incidence of dizziness, cough, and edema in patients with hypertension (32,33). Therefore, the dosage of sacubitril/valsartan should be closely monitored when being used. To sum up, sacubitril/valsartan, as the world’s first ARNI, is a new boon for hypertensive patients, especially middle-aged and elderly patients.
There were some limitations to this meta-analysis. Firstly, although the quality of included studies was generally high, the number of them was less than 10, which made it impossible to perform publication bias analysis. Secondly, the intervention duration of most of the included studies was short, with only 2 studies lasting 52 weeks, and there was insufficient evidence on the possible risks of long-term use of sacubitril/valsartan in the treatment of middle-aged and elderly hypertensive patients. Furthermore, the antihypertensive drugs used in the control group were relatively simple, only ARBs were used, and other first-line antihypertensive drugs such as CCB and ACEI were missing. Besides, in this study, subgroup analysis was only performed on different doses of sacubitril/valsartan, without subgroup analysis on factors such as race and gender of the included patients. Additionally, the number of included studies was small, with a total sample size of only 3,323 middle-aged and elderly patients with hypertension. Larger sample sizes need to be included in the future to further reflect the efficacy and safety of sacubitril/valsartan.
However, this meta-analysis was carried out at an appropriate time, because sufficient data had been accumulated for the examination of the meta-analysis method. A variety of strategies were used to identify the studies, rigorous criteria were used to include and evaluate the methodological quality of studies, and subgroup analysis and sensitivity analysis were performed. Hence, we provide the latest information in this area.
Conclusions
In this meta-analysis, sacubitril/valsartan has shown a better antihypertensive efficacy, while its safety is basically comparable to olmesartan, valsartan, and other antihypertensive drugs. Therefore, based on this result, sacubitril/valsartan can be used as a new approach in the clinical treatment of middle-aged and elderly patients with hypertension, which may bring better benefits to them, and is also very worthy of clinical application. However, although we adopted a strict approach, the limitations of the included studies made this meta-analysis less than perfect. Future studies with extensive, large samples, and well-designed follow-up are anticipated to update this analysis.
Acknowledgments
We sincerely appreciate and acknowledge Prof. Ying-Shi Piao from the Department of Pathology, Medical College of Yanbian University, Prof. Qing Li, Prof. Shu-Jie Yu, Prof. Su-Hua Li, and Prof. Bing-Yuan Wu from the Department of Cardiology, The Third Affiliated Hospital of Sun Yat-sen University, for their valuable efforts and suggestions on this paper.
Funding: This study was supported by the National Natural Science Foundation of China (No. 81860651), and the Science and Technology Department of Jilin Province (No. 20200201492JC).
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-503/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-503/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement:
References
- Muntner P, Carey RM, Gidding S, et al. Potential US Population Impact of the 2017 ACC/AHA High Blood Pressure Guideline. Circulation 2018;137:109-18. [Crossref] [PubMed]
- Lawes CM, Vander Hoorn S, Rodgers A, et al. Global burden of blood-pressure-related disease, 2001. Lancet 2008;371:1513-8. [Crossref] [PubMed]
- Wang Z, Chen Z, Zhang L, et al. Status of Hypertension in China: Results From the China Hypertension Survey, 2012-2015. Circulation 2018;137:2344-56. [Crossref] [PubMed]
- Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016;387:957-67. [Crossref] [PubMed]
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018;71:e13-e115. [PubMed]
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018;39:3021-104. [Crossref] [PubMed]
- Zhao D, Liu J, Wang M, et al. Epidemiology of cardiovascular disease in China: current features and implications. Nat Rev Cardiol 2019;16:203-12. [Crossref] [PubMed]
- Park JB, Kario K, Wang JG. Systolic hypertension: an increasing clinical challenge in Asia. Hypertens Res 2015;38:227-36. [Crossref] [PubMed]
- McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993-1004. [Crossref] [PubMed]
- Solomon SD, Zile M, Pieske B, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet 2012;380:1387-95. [Crossref] [PubMed]
- Ito S, Satoh M, Tamaki Y, et al. Safety and efficacy of LCZ696, a first-in-class angiotensin receptor neprilysin inhibitor, in Japanese patients with hypertension and renal dysfunction. Hypertens Res 2015;38:269-75. [Crossref] [PubMed]
- Shaddy R, Canter C, Halnon N, et al. Design for the sacubitril/valsartan (LCZ696) compared with enalapril study of pediatric patients with heart failure due to systemic left ventricle systolic dysfunction (PANORAMA-HF study). Am Heart J 2017;193:23-34. [Crossref] [PubMed]
- Supasyndh O, Wang J, Hafeez K, et al. Efficacy and Safety of Sacubitril/Valsartan (LCZ696) Compared With Olmesartan in Elderly Asian Patients (≥65 Years) With Systolic Hypertension. Am J Hypertens 2017;30:1163-9. [Crossref] [PubMed]
- Williams B, Cockcroft JR, Kario K, et al. Effects of Sacubitril/Valsartan Versus Olmesartan on Central Hemodynamics in the Elderly With Systolic Hypertension: The PARAMETER Study. Hypertension 2017;69:411-20. [Crossref] [PubMed]
- Cheung DG, Aizenberg D, Gorbunov V, et al. Efficacy and safety of sacubitril/valsartan in patients with essential hypertension uncontrolled by olmesartan: A randomized, double-blind, 8-week study. J Clin Hypertens (Greenwich) 2018;20:150-8. [Crossref] [PubMed]
- Huo Y, Li W, Webb R, et al. Efficacy and safety of sacubitril/valsartan compared with olmesartan in Asian patients with essential hypertension: A randomized, double-blind, 8-week study. J Clin Hypertens (Greenwich) 2019;21:67-76. [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: [PubMed]
- Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev 2019;10:ED000142. [Crossref] [PubMed]
- Wang TD, Tan RS, Lee HY, et al. Effects of Sacubitril/Valsartan (LCZ696) on Natriuresis, Diuresis, Blood Pressures, and NT-proBNP in Salt-Sensitive Hypertension. Hypertension 2017;69:32-41. [Crossref] [PubMed]
- Izzo JL Jr, Zappe DH, Jia Y, et al. Efficacy and Safety of Crystalline Valsartan/Sacubitril (LCZ696) Compared With Placebo and Combinations of Free Valsartan and Sacubitril in Patients With Systolic Hypertension: The RATIO Study. J Cardiovasc Pharmacol 2017;69:374-81. [Crossref] [PubMed]
- Schmieder RE, Wagner F, Mayr M, et al. The effect of sacubitril/valsartan compared to olmesartan on cardiovascular remodelling in subjects with essential hypertension: the results of a randomized, double-blind, active-controlled study. Eur Heart J 2017;38:3308-17. [Crossref] [PubMed]
- Robles NR, Macias JF. Hypertension in the elderly. Cardiovasc Hematol Agents Med Chem 2015;12:136-45. [Crossref] [PubMed]
- GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1659-724. Erratum in: Lancet 2017 Jan 7;389(10064):e1. [Crossref] [PubMed]
- Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2224-60. [Crossref] [PubMed]
- SPRINT Research Group. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med 2015;373:2103-16. [Crossref] [PubMed]
- Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019;394:1145-58. [Crossref] [PubMed]
- Nathisuwan S, Talbert RL. A review of vasopeptidase inhibitors: a new modality in the treatment of hypertension and chronic heart failure. Pharmacotherapy 2002;22:27-42. [Crossref] [PubMed]
- Shi J, Wang X, Nguyen J, et al. Sacubitril Is Selectively Activated by Carboxylesterase 1 (CES1) in the Liver and the Activation Is Affected by CES1 Genetic Variation. Drug Metab Dispos 2016;44:554-9. [Crossref] [PubMed]
- Daimon M, Konta T, Oizumi T, et al. Higher plasma renin activity is a risk factor for total mortality in older Japanese individuals: the Takahata study. Metabolism 2012;61:504-11. [Crossref] [PubMed]
- Zhao Y, Yu H, Zhao X, et al. The Effects of LCZ696 in Patients With Hypertension Compared With Angiotensin Receptor Blockers: A Meta-Analysis of Randomized Controlled Trials. J Cardiovasc Pharmacol Ther 2017;22:447-57. [Crossref] [PubMed]
- De Vecchis R, Soreca S, Ariano C. Anti-Hypertensive Effect of Sacubitril/Valsartan: A Meta-Analysis of Randomized Controlled Trials. Cardiol Res 2019;10:24-33. [Crossref] [PubMed]
- Li B, Zhao Y, Yin B, et al. Safety of the neprilysin/renin-angiotensin system inhibitor LCZ696. Oncotarget 2017;8:83323-33. [Crossref] [PubMed]
- Li Q, Li L, Wang F, et al. Effect and safety of LCZ696 in the treatment of hypertension: A meta-analysis of 9 RCT studies. Medicine (Baltimore) 2019;98:e16093. [Crossref] [PubMed]
(English Language Editor: J. Jones)




