
A multicenter, double-blind, placebo-controlled parallel study to evaluate the role of Yinhua Miyanling tablets in the prevention of bacterial biofilm formation on ureteral stents: a randomised trial
Introduction
Ureteral stents are widely used in urology. Ureteral stent-associated urinary tract infection (UTI) is a common but challenging problem to address. A study has demonstrated that the formation of bacterial biofilms, which not only greatly increase the risk of infection but are also closely associated with the occurrence and persistence of chronic infections, is closely related to ureteral stent-associated UTI (1). At present, the prevention and treatment of ureteral stent related urinary tract infection rely on antibiotics, but clinical practice shows that the application of antibiotics has poor effect on the prevention of ureteral stent related urinary tract infection and will lead to the occurrence of bacterial drug resistance. At the same time, the formation of bacterial biofilm makes the treatment of ureteral stent related urinary tract infection more difficult. Antibacterial traditional Chinese medicine can inhibit the adhesion of bacteria to the carrier, and the adhesion of bacteria is a key step in the formation of biofilm (2). These results indicate that traditional Chinese medicine (TCM) could inhibit the formation and development of biofilms as well as enhance the phagocytosis of white blood cells, regulate immunity, and have synergistic effects on antibiotics. Therefore, TCM has broad application value in the prevention and treatment of bacterial biofilms. In this study, Yinhua Miyanling tablet (YMT) was used as the active drug to observe the effect of an antibacterial TCM on the formation of ureteral stent biofilms through scanning electron microscopy (SEM). The effects of YMT during catheter placement were assessed via routine urine tests, urine cultures, body temperature and other indicators, and the effects of YMT on urinary tract irritation, pain, and other symptoms were observed. In the current situation of serious antimicrobial drug resistance in China, it is of great clinical significance to study antibacterial TCM for inhibiting biofilm formation and reducing the risk of clinical ureteral stent-related infection. We present the following article in accordance with the CONSORT reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-324/rc).
Methods
This study was designed to enroll patients who underwent ureteroscopic lithotripsy associated with indwelling ureteral stents at six centers between March 2019 and June 2020. The study was approved by Ethics Committees of Beijing Tongren Hospital, Capital Medical University (No. TRECKY2019-047), The First Affiliated Hospital of Guangzhou Medical University (No. 2019-36), and Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (No. 2019-S1047). The other 3 centers (Beijing Tiantan Hospital, Capital Medical University; Beijing Friendship Hospital, Capital Medical University; Civil Aviation General Hospital) were informed and agreed with the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and informed consent was taken from all the patients.
Study design and procedures
All enrolled patients were treated with ureteroscopic lithotripsy and ureteral stents. The inclusion criteria were as follows: the subject or his or her representative signed the informed consent form voluntarily; 18–65 years of age; urine culture was performed after the operation, and the urine culture was negative within one week before the operation; no fever or other symptoms of systemic infection present within two weeks before the operation; uncomplicated lithiasis evidenced by mild hydronephrosis and stones <2 cm in diameter; operation time ≤1 hour; unilateral surgical catheterization; no urinary tract obstruction after the operation; and the ureteral stent was in the proper position based on a kidney, ureter, and bladder (KUB) study on the second day after the operation. The exclusion criteria were as follows: antimicrobial agents were required during the period of indwelling ureteral stent after the operation (excluding perioperative prophylactic antibiotics); complicated with diseases that affect their immunity or general health, such as hypoproteinemia, cardiovascular and cerebrovascular diseases, liver, kidney and hematopoietic system diseases, an uncontrolled malignant tumor, and psychiatric disorders; pregnant or lactating women, or women who planned to be pregnant within three months after extubation; failure to comply with the study protocol; received any other study drug or participated in another interventional clinical trial within 3 months before screening; and other conditions, in the opinion of the investigator, not suitable for the patient to be enrolled in this study.
A statistician was responsible for all the data analyses. In this two-parallel study, random numbers were generated by the block randomization method using SAS 9.4 statistical software. The drug was packaged and coded by professionals who had nothing to do with this trial. The subjects were randomized strictly in accordance with the corresponding random number table. The patients in the experimental group began to take YMT (Z19991090, Jilin Huakang Pharmaceutical Co., Ltd., Jilin, China) 2 g qid orally from the first day after the operation, while the patients in the control group began to take dummy YMT (provided by Jilin Huakang Pharmaceutical Co., Ltd.) 2 g qid orally from the first day after the operation. This study was double blind in design. The test drug and placebo were allocated to the corresponding group appropriately. All data were entered into a database. The patients were unblinded following a blind review and data locking. The actual treatment of each patient was incorporated into the database corresponding to the experimental and control groups.
Demographic data, medical history, vital signs, and laboratory tests (hematology, serum chemistry, urinalysis, and urine culture) were collected 1–7 days before the operation. The body temperature and operation time were recorded on the day of the operation. On the first day after the operation, the body temperature was recorded, and the KUB study was repeated to determine the position of the ureteral stent. The study drugs were randomly allocated. The patients were instructed to take the drug for 14±3 days. The unused study drugs were recalled on day 14 (±3 days). The temperature was recorded. The ureteral stent symptom questionnaire (USSQ) was administered (3). Relevant laboratory tests (urinalysis and urine culture) were performed before removal of the ureteral stent. The specimen was collected for SEM when the ureteral stent was removed. Blood samples were collected for hematology tests and blood culture in case of fever (>38 ℃) during study treatment.
Laboratory procedures
The ureteral stent was removed via a flexible cystoscope under local anesthesia. A straight segment (2 cm) of the ureteral stent was cut for specimen preparation as follows: samples were fixed with 3% glutaraldehyde for 2 h; rinsed with 1 mol/L PB 3 times for 10 min each time; soaked in 1 mol/L PB and stored at 4 ℃; treated with 1% osmic acid for 1 h; rinsed with 1 mol/L PB 3 times for 10 min each time; soaked in 50%, 70%, 80%, and 90% alcohol for 10 min respectively; soaked in 100% alcohol for 15 min; and soaked in tert-butyl alcohol for 1 h and incubated in a 37 ℃ water bath. The film was sealed quickly and frozen at −20 ℃ for more than 2 hours, lyophilized overnight, and sputter coated in gold. SEM was used to evaluate the formation of bacterial biofilms and calculi on the surface of the ureteral stent.
Efficacy and safety evaluation
Bacterial biofilm formation on the ureteral stent (primary outcomes)
Polyurethane scaffolds were used consistently. The criteria for biofilm formation were as follows: a positive result was confirmed when the regulating film and basement membrane in the form of a three-layer structure (regulating film, basement membrane, and surface film) were detected. After the specimens were prepared at each center, they were transported to the central laboratory and evaluated by two doctors independently in a blinded manner. If there was any discrepancy in their results, a third doctor was the judge.
Evaluation of urinary tract symptoms during indwelling ureteral stents
The evaluation was based on the USSQ score and the following domains: body pain, general health, work performance, sexual matters, and additional problems.
Postoperative infectious complications
- Asymptomatic bacteriuria was defined as a positive urine culture (≥104 CFU/mL) before removal of the ureteral stent but without any clinical symptoms;
- Symptomatic UTI was defined as a positive urine culture (≥104 CFU/mL) associated with clinical symptoms such as frequent, urgent, and painful urination;
- High fever and chills were defined as body temperature >38.3 ℃ associated with chills and shivering; and
- Reproductive system infection, including acute epididymitis and prostatitis.
Safety evaluation
The clinical symptoms, signs, laboratory results of hematology tests and urinalysis and all adverse events (AEs) and serious adverse events (SAEs) were recorded and evaluated. AEs were rated as no, mild, moderate, or severe in severity. The correlation between AEs and drugs was evaluated in terms of the 6-level criteria (definitely related, likely related, possibly related, possibly unrelated, definitely unrelated, or unable to evaluate).
Statistical methods
The patients were included in the full analysis set (FAS), per protocol set (PPS), and safety set (SS) in this study as follows:
FAS: the ideal patient population for analysis on the principle of intent to treat. This dataset included all randomized patients who underwent at least one post dose efficacy evaluation after randomization by excluding subjects as minimally as possible on a reasonable basis.
PPS: included all the patients who complied well with the study protocol with the specified study data and did not take prohibited drugs during the study.
SS: included all randomized patients who received the study drug after randomization and underwent at least one safety assessment. The missing safety data were not carried forward. The incidence of AEs and adverse drug reactions was calculated by taking the number of patients in the SS as the denominator.
The FAS was used to compare baseline patient characteristics and all efficacy variables. The PPS was used to analyze the primary efficacy variable and in the supporting FAS analysis. The SS was used for the safety analysis.
SAS 9.4 software was used for the statistical analyses. Abnormally distributed measurement data are presented as the median and interquartile range (IQR) [M (p25–p75)] and were compared with the rank-sum test. Normally distributed measurement data are expressed as the mean ± standard deviation (SD) and were compared with the t-test. The enumeration data were compared with the chi-square test. The difference was considered statistically significant if P<0.05.
Results
A total of 211 patients were enrolled who underwent ureteroscopic lithotripsy associated with indwelling ureteral stents at six centers between March 2019 and June 2020 in this study, and 165 patients were included in the PPS group (86 in the control group and 79 in the experimental group). The participant flow chart is shown in Figure 1. Protocol violation was reported in 46 cases. The reasons for protocol violation were as follows: violation of the inclusion or exclusion criteria such as baseline urine culture, age, operation time, duration of an indwelling catheter (in 5 cases each); prohibited drugs in 3 cases; poor compliance (<70% prescribed dosage) in 12 cases; and lost to follow-up in 11 cases. There was no significant difference in baseline patient characteristics between the two groups (all P>0.05, Table 1).
Table 1
| Parameter | Control (n=86) | Experimental (n=79) | Statistic | P value |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 72 (83.7) | 58 (73.4) | 2.615 | 0.106 |
| Female | 14 (16.3) | 21 (26.6) | ||
| Age (mean ± SD) | 46.26±12.23 | 45.57±12.25 | −1.738 | 0.084 |
| BMI (mean ± SD) | 24.83±3.10 | 25.41±3.16 | −1.085 | 0.280 |
| Operation time (mean ± SD) | 27.85±15.71 | 28.91±14.10 | −0.456 | 0.649 |
| Duration of catheter indwelling (mean ± SD) | 13.84±2.99 | 13.67±3.23 | 3.306 | 0.508 |
BMI, body mass index; SD, standard deviation.
Bacterial biofilm formation on the ureteral stent
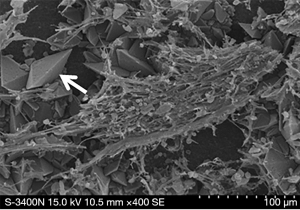
The prevalence of biofilm formation in the control group (47.0%) was significantly higher than that in the experimental group (22.7%, P=0.001). The USSQ score and urinary symptoms domain score were not significantly different between the two groups (all P>0.05). The formation of bacterial biofilms is illustrated in Figure 2.

Evaluation of urinary tract symptoms during indwelling ureteral stents
The total USSQ score and domain scores did not show a significant difference between the two groups (all P>0.05, Table 2), indicating that YMT did not reduce the incidence of symptoms related to ureteral stents in such patients.
Table 2
| Parameter | Control | Experimental | Statistic | P value |
|---|---|---|---|---|
| USSQ | 25.80±6.28 | 26.25±7.50 | −0.413 | 0.680 |
| Body pain | 13.11±7.92 | 13.08±7.88 | 0.027 | 0.978 |
| General health | 12.19±3.93 | 12.13±3.66 | 0.101 | 0.920 |
| Work performance | 7.24±4.00 | 7.56±4.13 | −0.499 | 0.618 |
| Sexual matters | 2.00 (1.00, 5.00) | 2.00 (1.00, 2.00) | −0.714 | 0.475 |
| Additional problems | 10.63±2.61 | 10.97±2.77 | −0.820 | 0.414 |
| Total | 74.70±19.50 | 73.49±18.98 | 0.401 | 0.689 |
USSQ, ureteral stent symptom questionnaire.
Postoperative infectious complications
There were significantly more patients with symptomatic UTI in the control group (12.9%) than in the experimental group (2.6%, P=0.017). The incidence of other complications (asymptomatic bacteriuria, high fever and chills, low fever, and reproductive system infection) did not show a significant difference between the two groups (all P>0.05, Table 3).
Table 3
| Parameter | Stratum | Control, n (%) | Experimental, n (%) | Statistic | P value |
|---|---|---|---|---|---|
| Asymptomatic bacteriuria | No | 81 (82.4) | 76 (97.4) | 5.201 | 0.123 |
| Yes | 4 (4.7) | 0 (0) | |||
| Symptomatic urinary tract infection | No | 74 (87.1) | 74 (97.4) | 5.746 | 0.017 |
| Yes | 11 (12.9) | 2 (2.6) | |||
| High fever and chills | No | 70 (100.0) | 53 (98.1) | 1.673 | 0.435 |
| Yes | 0 | 1 (1.9) | |||
| Acute epididymitis (males only) | No | 70 (100.0) | 54 (100.0) | – | – |
| Yes | 0 | 0 | |||
| Acute prostatitis (males only) | No | 70 (100.0) | 53 (98.1) | 1.673 | 0.435 |
| Yes | 0 | 1 (1.9) |
Encrustation of the surface of the ureteral stent
Encrustation of the surface of the ureteral stent was found in 72.3% of the patients in the control group and 65.3% of the patients in the experimental group (P=0.345). Encrustation of the surface of the ureteral stent is illustrated in Figure 3.
Change in white blood counts (WBCs) in the urine after treatment (flow cytometry)
After treatment, the WBC count in the urine showed a significant difference between the two groups (P<0.001, Table 4).
Table 4
| Parameter | Control | Experimental | Statistic | P value |
|---|---|---|---|---|
| WBCs in the urine [M (P25–P75)] | ||||
| Pretreatment | 12.90 (4.00, 29.80) | 17.91 (6.72, 69.98) | −2.017 | 0.044 |
| Posttreatment | 81.00 (39.50, 201.05) | 45.27 (13.90, 92.37) | −3.779 | <0.001 |
| WBCs in the urine after treatment by microscopy, n (%) | ||||
| Decrease/stable | 29 (35.4) | 45 (62.5) | 11.308 | 0.001 |
| Increase | 53 (64.6) | 27 (37.5) |
WBC, white blood count.
Adverse drug reactions
Mild adverse drug reactions were reported in 2 (2.5%) patients in the control group and 4 (4.7%) patients in the experimental group (P=0.683). The adverse drug reactions included gastrointestinal reactions and nervous system reactions.
Discussion
The formation of bacterial biofilms on the surface of ureteral stents can result in a series of adverse effects, such as bacteriuria and urinary sepsis (4). In order to prevent the formation of bacterial biofilms, urologists have made many attempts to develop and apply new coatings, new materials, and new technologies. However, there are still unmet medical needs in this aspect, although some progress has been made (5). Antimicrobial agents can inhibit the formation of biofilms to some extent, but it is not realistic to use antimicrobial agents extensively to prevent the formation of biofilms, which will inevitably lead to problems related to antimicrobial drug abuse and bacterial resistance. Chinese researchers have explored strategies for the prevention of biofilm formation with antibacterial TCMs, but multicenter randomized controlled studies are still lacking. Therefore, we designed this study to address this issue.
TCM has unique advantages in the treatment of infectious diseases. Modern pharmacological research has shown that TCM cannot only improve the body’s immunity but also reduce bacterial resistance to antibiotics (6). YMT is a pure TCM preparation composed of Lonicerae Japonicae Flos, Scutellaria barbata, and Polygonum aviculare. It works by eliminating heat and toxins in the body, evidenced by diuresis and dehydration, and inhibiting the growth of Escherichia coli, the main pathogen of UTIs. Basic study has demonstrated that the drug may interfere with or destroy the synthesis of the bacterial cell wall or destroy the large molecular weight protein in the bacterial cell, resulting in a change in the cell ultrastructure, which leads to inhibition or killing of the bacteria (7). In vitro antimicrobial susceptibility testing showed that the 95% ethanol extract from YMT had potent antibacterial activity against the reference strain ATCC 25922 and 20 clinical strains of E. coli isolated from UTI cultures (8).
The results of this study show that YMT can better inhibit the formation of bacterial biofilms on ureteral stents than placebo, providing a new solution for the prevention and treatment of bacterial biofilm formation in such patients. One study has shown that YMT can significantly inhibit the formation of E. coli biofilms because it can downregulate gene expression of the flu gene (flagellin) and polysaccharide glmU gene in E. coli biofilms. YMT may inhibit bacterial growth by inhibiting the expression of the flu and glmU genes and thus inhibiting the production of adhesion proteins and extracellular polysaccharides in the early stage of biofilm formation, thus inhibiting the formation and maturation of biofilms (9).
This study showed that YMT could significantly reduce the incidence of symptomatic UTI in patients with ureteral stents compared with placebo, indicating that YMT has a good antimicrobial effect in patients with indwelling ureteral stents after stone surgery. Antimicrobial susceptibility testing was performed by the Institute of Clinical Pharmacology, Peking University for 336 clinical strains collected from 18 hospitals in China in the last 3 years. The results showed that YMT had an inhibitory effect on E. coli, Enterococcus, Staphylococcus epidermidis, and other Gram-positive cocci. Its antibacterial activity was independent of the production of extended spectrum beta-lactamases (ESBLs), methicillin resistance, or ciprofloxacin resistance (10).
The change in WBC count in the urine after treatment showed a significant difference between YMT and placebo. YMT-treated patients had a significantly lower prevalence of increased WBCs in the urine than placebo-treated patients, indicating that YMT has good anti-inflammatory effects. YMT plays an anti-inflammatory role by inhibiting lipopolysaccharide-induced inflammatory corpuscles in human peripheral blood monocytes (11).
A considerable number of patients with indwelling ureteral stents will develop varying degrees of urinary tract symptoms, which affect urination and quality of life (12). YMT also has the effect of clearing away heat and toxins, detumescence, killing pain, and diuresis. In this study, we also examined the effect of YMT on urinary tract symptom relief during indwelling ureteral stents. The results showed that there was no significant difference between placebo and YMT, indicating that YMT could not reduce urinary tract symptoms in such patients. The decreased urinary tract symptoms caused by ureteral stents may be related to stimulation of the bladder triangle. Body pain may be related to ureteral spasm and urinary reflux. However, sexual dysfunction may be due to urinary tract symptoms and physical discomfort, which affect an individual’s sexual desire and self-confidence. The role of YMT in improving these symptoms is unclear.
In addition to biofilm formation, encrustation is another important clinical problem related to ureteral stents. Biofilm formation and encrustation, the potential mechanisms of which are related to bacterial adhesion and crystal deposition, are interdependent. In this study, we also explored the effect of YMT on the encrustation of ureteral stents. The results showed that YMT had no effect on inhibiting the encrustation of ureteral stents. The process of encrustation starts from the adhesion of the host protein in the urine to the surface of the ureteral stents, and the stents provide a surface for the bacteria in the urine to adhere. Bacteria form biofilms on the surface of stents (13). For urease-negative bacteria, the extracellular polysaccharide matrix of biofilms makes more crystals remain in the urine. For urease-positive bacteria such as Proteus mirabilis, urease decomposes urea into carbon dioxide and ammonia, and excess ammonia increases the pH value of urine, which can lead to the precipitation of magnesium ammonium phosphate and crystal formation (14). One study has shown that crystals form 11–17 days after a ureteral stent is placed in the body (15). Crystal formation is positively correlated with the indwelling duration (16) and inversely proportional to the diameter of the ureteral stent (17). At present, it has not been shown that YMT can promote the excretion of crystals from the urine. The antibacterial activity of YMT against Proteus mirabilis has not been well studied. Therefore, YMT has no effect on the prevention of encrustation of ureteral stents. Early removal of catheters is the best method for the prevention of encrustation.
Conclusions
This is the first multicenter, randomized, controlled study to confirm the inhibitory effect of YMT on biofilm formation on ureteral stents after an operation to remove calculus. YMT has both anti-infective and anti-inflammatory effects; it can significantly reduce the incidence of symptomatic UTI in patients with indwelling ureteral stents and reduce the WBC count in the urine. However, YMT has no effect on relieving symptoms or preventing stent encrustation during indwelling ureteral stents.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-324/rc
Trial Protocol: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-324/tp
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-324/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-324/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committees of Beijing Tongren Hospital, Capital Medical University (No. TRECKY2019-047), The First Affiliated Hospital of Guangzhou Medical University (No. 2019-36), and Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (No. 2019-S1047). The other 3 centers (Beijing Tiantan Hospital, Capital Medical University; Beijing Friendship Hospital, Capital Medical University; Civil Aviation General Hospital) were informed and agreed with the study. Informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Scotland KB, Lo J, Grgic T, et al. Ureteral stent-associated infection and sepsis: pathogenesis and prevention: a review. Biofouling 2019;35:117-27. [Crossref] [PubMed]
- Yu GY, Xie YM, Gao N, et al. Clinical application evaluation of clinical practice guideline on traditional Chinese medicine therapy alone or combined with antibiotics for uncomplicated lower urinary tract infection. Zhongguo Zhong Yao Za Zhi 2018;43:4746-52. [PubMed]
- Joshi HB, Newns N, Stainthorpe A, et al. Ureteral stent symptom questionnaire: development and validation of a multidimensional quality of life measure. J Urol 2003;169:1060-4. [Crossref] [PubMed]
- Altunal N, Willke A, Hamzaoğlu O. Ureteral stent infections: a prospective study. Braz J Infect Dis 2017;21:361-4. [Crossref] [PubMed]
- Khoddami S, Chew BH, Lange D. Problems and solutions of stent biofilm and encrustations: A review of literature. Turk J Urol 2020;46:S11-8. [Crossref] [PubMed]
- Yu GY, Tian ZJ, Sun Y, et al. Review on advantages and evidence of treating and preventing urinary tract infection in traditional Chinese medicine. Zhongguo Zhong Yao Za Zhi 2017;42:1439-48. [PubMed]
- Sun T, Li XD, Hong J, et al. Inhibitory Effect of Two Traditional Chinese Medicine Monomers, Berberine and Matrine, on the Quorum Sensing System of Antimicrobial-Resistant Escherichia coli. Front Microbiol 2019;10:2584. [Crossref] [PubMed]
- Hu Y, Wang JH, Zhang XT, et al. Preliminary study of antibacterial mechanism of Yinhua Miyan Ling Tablet on Escherichia coli by KCs method. Journal of Traditional Chinese Medicine University of Hunan 2012;32:3-4.
- Sai JY, Hu Y, Zhang C, et al. Influence of ethanol extract of Yinhua Miyanling Tablets in ultrastructure of Escherichia coli and its antibacterial mechanism. Journal of Jilin University 2014;40:117-20. (Medicine Edition).
- Li Y, Lv Y, Liu J, et al. In vitro activity of Yinhua Miyan Ling. Chin J Clin Pharmacol 2015:279-82.
- Zhang WT, Miao RP, Zhao QH, et al. Clinical and fundamental research Yinhua Miyanling Tablets in treating urinary tract infection. Zhongguo Zhong Yao Za Zhi 2019;44:2403-10. [PubMed]
- Betschart P, Zumstein V, Buhmann MT, et al. Symptoms Associated With Long-term Double-J Ureteral Stenting and Influence of Biofilms. Urology 2019;134:72-8. [Crossref] [PubMed]
- Tomer N, Garden E, Small A, et al. Ureteral Stent Encrustation: Epidemiology, Pathophysiology, Management and Current Technology. J Urol 2021;205:68-77. [Crossref] [PubMed]
- Broomfield RJ, Morgan SD, Khan A, et al. Crystalline bacterial biofilm formation on urinary catheters by urease-producing urinary tract pathogens: a simple method of control. J Med Microbiol 2009;58:1367-75. [Crossref] [PubMed]
- Wollin TA, Tieszer C, Riddell JV, et al. Bacterial biofilm formation, encrustation, and antibiotic adsorption to ureteral stents indwelling in humans. J Endourol 1998;12:101-11. [Crossref] [PubMed]
- Wang L, Zhang S, Keatch R, et al. In-vitro antibacterial and anti-encrustation performance of silver-polytetrafluoroethylene nanocomposite coated urinary catheters. J Hosp Infect 2019;103:55-63. [Crossref] [PubMed]
- Kawahara T, Ito H, Terao H, et al. Ureteral stent encrustation, incrustation, and coloring: morbidity related to indwelling times. J Endourol 2012;26:178-82. [Crossref] [PubMed]



