
The efficacy and safety of tacrolimus and entecavir combination therapy in the treatment of hepatitis B virus-associated glomerulonephritis: a multi-center, placebo controlled, and single-blind randomized trial
Introduction
Hepatitis B virus (HBV) infection is a major global public health concern, with a global prevalence of about 3.5%. Worldwide, approximately 248 million individuals carried the hepatitis B surface antigen (HBsAg) (1,2). Despite the availability of an effective HBV vaccine, in China, there are about 70 million people who carry the HBsAg, with a prevalence of 5–6% (3,4). The incidence of hepatitis B virus-associated glomerulonephritis (HBV-GN) is 3–5% in patients with chronic HBV infection, and this is the most common extrahepatic manifestation of HBV infection (5). HBV-GN is more prevalent in children compared to adults, and males compared to females. However, contrary to children, spontaneous remission is relatively rare in adults, with approximately 30% of HBV-GN patients progressing to end-stage renal disease (ESRD) and 10% requiring maintenance dialysis therapy (6,7).
The 2021 Kidney Disease: Improving Global Outcomes (KDIGO) guidelines recommend the use of nucleos(t)ide analogues for the treatment of HBV-GN. Entecavir (ETV) is the first-line antiviral drug with good efficacy and low drug resistance. However, proteinuria response was lower in HBV-GN patients with high proteinuria treated with antiviral therapy alone (8). In addition, the use of immunosuppressive agents for the treatment of HBV-GN remains controversial due to following concerns: the activation of HBV DNA replication, liver damage, and renal impairment (9). Although glucocorticoids can relieve urinary protein, they have little effect on virus clearance and may cause HBV replication and explosive hepatitis (10). This could be because glucocorticoids can interact with glucocorticoid-responsive elements in the HBV genome, causing HBV gene expression to be upregulated (11).
Tacrolimus (TAC), a calcium phosphatase inhibitor, prevents HBV from infecting hepatocytes via interfering with its binding to sodium taurocholate cotransporting polypeptide (NTCP), and protects renal function through inhibiting calcineurin inhibitors (12,13). Furthermore, TAC can act directly on podocytes, stabilizing the actin cytoskeleton and inhibiting podocyte apoptosis, reducing urinary protein (14). Our previous retrospective study discovered that TAC in combination with entecavir has good efficacy in the treatment of HBV-GN and can significantly reduce urinary protein (15). However, the limited data on the combination treatment of TAC and entecavir in HBV-GN patients are only based upon observations. The efficacy and safety of the combination therapy consisting of TAC and entecavir in HBV-GN patients remains unclear. Therefore, we conducted a prospective, randomized, controlled, multicenter, single-blinded, two-parallel study to evaluate the efficacy and safety of the combination therapy of TAC and entecavir in the treatment of HBV-GN patients in China. We present the following article in accordance with the CONSORT reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-328/rc).
Methods
Study design and participants
This trial [Tacrolimus Combined with Entecavir on HBV Associated Glomerulonephritis (HBV-GN) (TOHBVGN)] was conducted in Guangzhou, Guangdong Province, China, with subjects recruited from 6 university hospitals in 4 Chinese provinces (Guangdong, Hunan, Hebei, and Jilin). Patients were enrolled in Guangdong Provincial People’s Hospital, Affiliated Hospital of Guangdong Medical College, Second Hospital of Jilin University, Second Hospital of Hebei Medical University, Xiangya Hospital of Central South University, and The First Affiliated Hospital of Nanhua. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Guangdong Provincial People’s Hospital Research Ethics Committee [No. GDREC2015287H(R1)]. Written informed consents were obtained from all patients who agreed to participate and the study was approved by institutional review board at all participating institutions.
All enrolled patients satisfied the following inclusion criteria: (I) patients aged 18 to 70 years, male and female; (II) patients with documented evidence of HBV-GN diagnosis by HBV antigen deposition in renal puncture biopsy tissue and serological HBV infection (positive HBsAg, HBeAg, or HBV DNA titer >10^3 IU/mL in the serum) lasting for six months; (III) patients did not receive antiviral therapy in the past six months; (III) patients had at least 2 separate tests showing 24-hour proteinuria (24 h UP) >3.0 g, and urine protein to creatinine ratio (UPCR) >3,000 mg/g·cr; (V) Patients did not receive glucocorticoid and immunosuppressive therapy within the previous 2 weeks.
The exclusion criteria were as follows: (I) idiopathic membranous nephropathy (MN), systemic lupus erythematosus, malignancy, diabetes mellitus, severe infection, or any other systemic diseases known to be associated with secondary MN; (II) patients with a history of severe heart disease, cerebrovascular disease, allergy to TAC or entecavir; (III) patients with an eGFR <30 mL/(min·1.73 m2) or tubular atrophy or interstitial fibrosis ≥50% in renal biopsies; (IV) patients with innate or acquired immunodeficiency, liver cirrhosis, pregnancy, breast feeding, or other active infection such as cytomegalovirus (CMV), tuberculosis, hepatitis A virus (HAV), hepatitis C virus (HCV), hepatitis D virus (HDV).
Randomization and masking
Eligible subjects were randomly assigned in a 1:1 ratio by computer-generated random numbers to two different groups and received either tacrolimus combined with entecavir (TAC+ETV) treatment or entecavir combined with placebo (ETV). Random envelopes containing random codes were generated before the study and distributed to patients according to the time of enrollment. Odd or even random numbers determined the allocation of treatment groups. An investigator who was not involved in the randomization procedure prepared all opaque, sealed envelopes containing the computer-generated random numbers to ensure that the treatment was concealed. To achieve single-blindness, the placebo and TAC did not differ in dosage form, appearance, taste, or packaging, and patients were not aware of the actual therapeutic drug. Patients were unaware of the treatment allocation.
Procedures
All patients received oral entecavir 0.5 mg tablet each night for the first 2 weeks, then they were treated with tacrolimus and entecavir or entecavir with a matching placebo of tacrolimus for the next 24 weeks. In the treatment group, the TAC+ETV group, patients received 0.05–0.1 mg/(kg·d) tacrolimus every 12 hours for a day and 0.5 mg entecavir every night. Subsequent doses were adjusted to achieve serum trough levels between 5 and 10 ng/mL. In the control group, the ETV group, patients were given tacrolimus placebo 0.05–0.1 mg/(kg·d) every 12 hours for a day and 0.5 mg entecavir every night. During the therapy, angiotensin converting enzyme inhibitor (ACEI) or angiotensin receptor antagonist (ARB), antiplatelet agents, diuretics etc. were administered according to individual conditions and none of the patients were treated with glucocorticoids.
All patients completed 26 weeks of follow-up. Adverse events were assessed at each study visit. Estimated glomerular filtration rate (eGFR) was performed in accordance with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) guidelines.
Outcome measures
Patients were assessed at 6, 10, 14, 18, 22, and 26 weeks after treatment began. The primary outcome measures were the remission rate of proteinuria at week 26, including complete remission (CR) and partial remission (PR). CR was defined as 24 h UP <0.3 g or UPCR <300 mg/g·cr plus stable renal function (eGFR >50 mL/min/1.73 m2). PR was defined as 24 h UP between 0.3 g to 3.0 g or UPCR between 300 mg/g·cr to 3,000 mg/g·cr plus 50% lower than baseline proteinuria and stable renal function. The secondary outcome measures were the remission rate of 24 h UP at week 14, renal function impairment, the incidence of serum HBV DNA negative conversion and serum HBV DNA breakthrough, the relapse rate of proteinuria, the incidence of acute kidney injury and ESRD (eGFR <15 mL/min/1.73 m2 or start kidney replacement therapy), the change of serum albumin (sALB), eGFR, serum HBV DNA titer, fasting blood glucose (FBG), aspartate aminotransferase (AST) and alanine aminotransferase (ALT), and the incidence of abnormal liver function [ALT or AST >2 times the upper limit value (ULN)], elevated FBG (>6.1 mmol/L) and other adverse events.
The renal function impairment was defined as serum creatinine levels increased by 50% or more from baseline or eGFR levels decreased by 50% or more from baseline. The serum HBV DNA negative conversion: the serum HBV DNA titer >500 IU/mL then lower than 500 IU/mL or undetectable. The serum HBV DNA breakthrough: the serum HBV DNA titer increased by >1 lg IU/mL compared with the lowest value in the treatment, or the blood HBV DNA titer was lower than the test value then higher than the test value. The relapse rate of proteinuria: 24 h UP >3.0 g or UPCR >3,000 mg/g after CR or PR.
Statistical analysis
The initial sample size was calculated based on the proteinuria remission rate observed in two treatments in our preliminary clinical study (15). To establish a difference between the two groups, a sample size of 112 patients was calculated to achieve 90% power and a two-sided significance level of 0.05.
All patients who assigned to treatment were included in the efficacy and safety analyses. SPSS (version 25) software was used for all analyses. Intention-to-treat (ITT) and per-protocol (PP) analyses were applied to compare intergroup differences. Missing data were imputed using the last observation carried forward (LOCF) method. Patients with missing values at baseline were excluded from analyses of change from baseline efficacy and safety outcomes. COX regression analysis based on the Least Significance Difference were used to compare the difference of proteinuria remission rate and serum HBV DNA negative rate between the two groups. The rate of HBV DNA virology breakthrough, abnormal ALT, abnormal AST, fasting glucose elevation and other adverse events were compared between the two groups using chi-square analysis. Statistical significance was determined by examining mean differences between pre- and post-intervention using Repeated ANOVA with Bonferroni posttest. And baseline factors that differed by ALT, defined as a P value <0.05, were included as covariates. The Kaplan-Meier analysis based on log-rank test was used to obtain the cumulative remission rate curve during the follow-up period. All reported P values are two-sided. The level of significance was set to P <0.05 for all analyses.
Results
Patients were enrollment between June 2016 and April 2019, and the 26-week follow-up period concluded in December 2019. There were 146 patients diagnosed with HBV-GN, but only 33 patients met the criteria for the study (Figure 1). Thus, 33 patients underwent randomization. In the TAC+ETV group (n=16), 2 patients withdrew consent and the remaining patients received TAC and ETV combination therapy. In the ETV group, 17 patients received ETV and placebo therapy, and 1 patient dropped out due to severe adverse events. Two of the 14 patients in the TAC+ETV group and 2 of the 17 patients in the ETV group were lost to follow-up. In total, 12 patients in the TAC+ETV group and 14 patients in the ETV group completed the treatment.
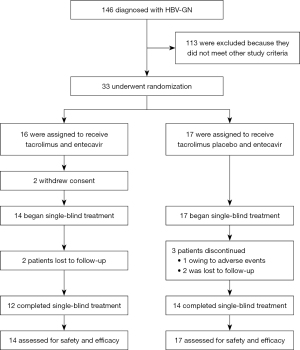
The baseline demographic and clinical characteristics among the two groups were comparable, with the exception of ALT levels (Table 1). Thus, in both the statistical analysis plans, ALT was included in the adjusted analyses of COX regression and Repeated ANOVA. A total of 90.3% (28/31) of patients were pathologically diagnosed with MN, of whom 12 patients were in the TAC+ETV group and 16 patients were in the ETV group. In total, 31 patients received treatment and were analyzed with the ITT protocol, but PP analyses excluded 2 patients in the TAC+ETV group and 3 patients in the ETV group due to incomplete treatment.
Table 1
| Characteristic | TAC+ETV group (n=14) | ETV group (n=17) |
|---|---|---|
| Age (years) | 44.0±14.2 | 43.9±9.9 |
| Male sex (%) | 12 (85.7) | 14 (82.4) |
| 24 h UP (g) | 7.88±3.62 | 6.20±3.92 |
| UPCR (mg/g·cr) | 6,222±3,590 | 4,789±5,276 |
| Scr (mmol/L) | 84.3±27.7 | 90.2±36.6 |
| eGFR (mL/min·1.73 m2) | 95.0±26.1 | 89.5±26.2 |
| Serum albumin (g/L) | 25.1±5.39 | 29.4±8.45 |
| Serum HBV DNA titer (log10, copies/mL) | 4.11±2.24 | 5.44±2.00 |
| Uria HBV DNA titer (log10, copies/mL) | 2.98±0.42 | 2.64±1.03 |
| ALT (U/L) | 23.9±16.6 | 46.8±33.3 |
| AST (U/L) | 32.2±18.2 | 44.7±27.7 |
| FBG (mmol/L) | 5.36±1.40 | 4.90±0.60 |
| Cholesterol (mmol/L) | 7.82±2.38 | 7.18±1.64 |
| Triglyceride (mmol/L) | 3.28±2.25 | 2.82±1.63 |
| HBsAg (%) | 14 (100.0) | 17 (100.0) |
| HBeAg (%) | 8 (57.1) | 14 (82.4) |
| HBcAb (%) | 14 (100.0) | 9 (52.9) |
| Pathology | ||
| Membranous nephropathy (MN, %) | 12 (85.7) | 16 (94.1) |
| Ig A nephropathy (IgAN, %) | 0 | 1 (5.9) |
| Mesangial proliferative glomerulonephritis (MsPGN, %) | 1 (7.1) | 0 |
| Focal segmental glomerulosclerosis (FSGS, %) | 1 (7.1) | 0 |
| Minimal change disease (MCD, %) | 0 | 0 |
| Membrane proliferative glomerulonephritis (MPGN, %) | 0 | 0 |
Date are presented as n (%) or mean ± standard deviation (SD). TAC, tacrolimus; ETV, entecavir; HBV, hepatitis B virus; ALT, alanine aminotransferase; AST, aspartate aminotransferase; UPCR, urine protein to creatinine ratio; 24 h UP, 24-hour proteinuria; FBG, fasting blood-glucose; HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B e antigen; HBcAb, hepatitis B core antibody.
Primary and secondary efficacy endpoints
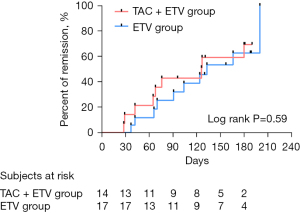
In the ITT analyses, after adjusting for baseline ALT, 42.9% (6/14) of patients in the TAC+ETV group reached remission (CR and PR) compared to 41.2% (7/17) in the ETV group at week 14 (P=0.23). At week 26, 64.3% (9/14) in the TAC+ETV group compared to 58.8% (10/17) in the ETV group achieved remission (CR/PR) (P=0.38). In the PP analyses, after adjusting for baseline ALT, 50.0% (6/12) of patients in the TAC+ETV group reached remission (CR/PR) compared to 50.0% (7/14) in the ETV group at week 14 (P=0.18), and at week 26, 75.0% (9/12) in the TAC+ETV group compared to 71.4% (10/14) in the ETV group reached remission (CR/PR) (P=0.32; Table 2). The results from the PP analyses the remission rates at week 14 and week 26 were consistent, although not statistically significant. However, in the ITT and PP analyses, two patients in the TAC+ETV group reached CR and none of the patients in the ETV group achieved CR. The median time to remission (CR/PR) in the TAC+ETV group was 126 days compared to 133 days in the ETV group (log rank test, P=0.59; Figure 2).
Table 2
| Parameter | TAC+ETV group (n=14) (%) | ETV group (n=17) (%) | P value |
|---|---|---|---|
| Primary efficacy endpoints | |||
| Remission rate of proteinuria at 26 weeks (ITT analyses) | 9 (64.3) | 10 (58.8) | 0.38 |
| Remission rate of proteinuria at 26 weeks (PP analyses) | 9 (75.0) | 10 (71.4) | 0.32 |
| Secondary endpoints | |||
| Remission rate of proteinuria at 13 weeks (ITT analyses) | 6 (42.9) | 7 (41.2) | 0.23 |
| Remission rate of proteinuria at 13 weeks (PP analyses) | 6 (50.0) | 7 (50.0) | 0.18 |
| Serum HBV DNA negative conversion | 5/8 (62.5) | 12/15 (80.0) | 0.19 |
| Relapse rate of proteinuria | 4/9 (44.4) | 6/10 (60.0) | 0.45 |
| Renal function impairment | 0 | 1 (5.9) | 1.00 |
| ESRD | 0 | 0 | – |
| AKI rate | 0 | 0 | – |
| Safety efficacy | |||
| HBV DNA virologic breakthrough | 0/11 (0.0) | 4/14 (28.6) | 0.11 |
| Elevated ALT (2ULN) | 1/13 (7.7) | 1/9 (11.1) | 1.00 |
| Elevated AST (2ULN) | 0/11 (0.0) | 2/10 (20.0) | 0.22 |
| Elevated fasting blood-glucose | 6 (42.9) | 4 (23.5) | 0.44 |
| Severe edema (SAE) | 0 | 1 (5.9) | 1.00 |
| Muscle spasm | 1 (7.1) | 0 | 0.45 |
P value: TAC+ETV group vs. ETV group. Proteinuria relapse: 24 h UP >3.0 g or UPCR >3,000 mg/g after CR or PR. TAC, tacrolimus; ETV, entecavir; AKI, acute kidney injury; SAE, serious adverse event; ESRD, end-stage renal disease; ULN, upper limit value; ITT, Intention-To-Treat; PP, Per-Protocol; CR, complete remission; PR, partial remission; HBV, hepatitis B virus; ALT, alanine transferase; AST, aspartate transferase.
In patients who achieved remission (CR or PR), 44.4% (4/9) in the TAC+ETV group and 60% (6/10) in the ETV group relapsed (P=0.45; Table 2). In patients whose serum HBV DNA were greater than 500 IU/mL at baseline, 62.5% (5/8) of patients in the TAC+ETV group and 80% (12/15) in the ETV group achieved serum HBV DNA negative conversion during the follow-up period (P=0.19).
Other efficacy endpoints
In the ITT analysis, 24 h UP decreased from 7.88±3.62 g at baseline to 5.25±4.29 g at week 26 in the TAC+ETV group and from 6.08±3.83 to 4.66±4.91 g in the ETV group. There was no significant difference in the decreased level of 24 h UP between the two groups (2.63±6.33 vs. 1.42±4.34 g, P=0.55; Figure 3A). In the PP analysis, 24h UP decreased from 8.08±3.57 g at baseline to 4.84±4.40 g at week 26 in the TAC+ETV group and from 6.71±3.93 to 5.03±5.35 g in the ETV group. There was no significant difference in the decreased level of 24 h UP between the two groups (3.24±6.13 vs. 1.68±4.77 g, P=0.50).
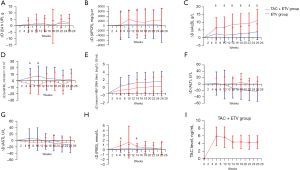
In the ITT analysis, UPCR reduced from 6,222±3,590 mg/g·cr at baseline to 3,875±3,601 mg/g·cr at week 26 in the TAC+ETV group and from 4,789±4,936 to 4,631±3,669 mg/g·cr in the ETV group (reduced 2,348±4,735 vs. 158±7,060 mg/g·cr, P=0.66; Figure 3B). In the PP analysis, UPCR decreased from 5,850±3,202 mg/g·cr at baseline to 2,933±2,905 mg/g·cr at week 26 in the TAC+ETV group and from 4,914±5,453 to 4,727±4,043 mg/g·cr in the ETV group (decreased 2,918±4,320 vs. 187±7,832 mg/g·cr, P=0.60).
ITT analysis showed a significant increase in sALB in both groups, with a greater increase in the TAC+ETV group at week 6, 10, 14, 18, 22, and 26 than in the ETV group (at week 6, P=0.006; at week 22, P=0.001; all others, P<0.001; Figure 3C). The sALB in the TAC+ETV group increased from 25.1±5.39 g/L at baseline to 36.1±6.16 g/L at week 26, while the ETV group increased from 29.4±8.45 to 33.2±8.28 g/L (increased 11.1±7.30 vs. 3.81±5.09 g/L, P<0.001). PP analysis also showed a significant increase in sALB in both groups, with a greater increase in the TAC+ETV group at week 6, 10, 14, 18, 22, and 26 than in the ETV group (at week 6, P=0.004; at week 18, P=0.003; at week 22, P=0.002; all others, P<0.001). And the increased level in sALB from baseline to week 26 in the TAC+ETV group was 12.4±6.82 g/L, while the ETV group was 4.09±5.09 g/L (P<0.001).
Changes in eGFR from baseline were statistically significant between the two groups in the ITT and PP analyses at week 6 and week 10 (at week 6 P=0.012, at week 10, P=0.002, in the ITT analysis; at week 6 P=0.011, at week 10, P=0.006, in the PP analysis), where TAC+ETV group decreased from baseline and ETV group increased from baseline. The most significant change in eGFR between the two groups occurred at week 10, while the decreased level from baseline to week 10 in the TAC+ETV group was 7.20±13.8 mL/min/1.73 m2 and the increased level in the ETV group was 7.74±15.1 mL/min/1.73 m2 in the ITT analysis (P=0.012; Figure 3D). In the PP analysis, the decreased level from baseline to week 10 in the TAC+ETV group was 6.63±14.2 mL/min/1.73 m2 and the increased level in the ETV group was 7.03±13.6 mL/min/1.73 m2 (P=0.006).
At other follow-up time points, ITT and PP analysis showed no significant difference in eGFR changes between the two groups (P>0.05). In the ITT analysis, eGFR in the TAC+ETV group changed from 95.0±26.1 mL/min/1.73 m2 at baseline to 95.3±27.5 mL/min/1.73 m2 at week 26, while decreased from 89.5±26.2 mL/min/1.73 m2 at baseline to 87.2±24.6 mL/min/1.73 m2 at week 26 in the ETV group (P>0.05). In PP analysis, eGFR in the TAC+ETV group increased from 101±21.0 mL/min/1.73 m2 at baseline to 103±17.5 mL/min/1.73 m2 at week 26, while decreased from 89.6±26.8 mL/min/1.73 m2 at baseline to 84.1±26.0 mL/min/1.73 m2 at week 26 in the ETV group (P>0.05).
The serum HBV DNA titer decreased in both groups, according to ITT and PP analyses, but the decreased level was no statistical difference between them (P=0.31, in the ITT analyses; P=0.09, in the PP analyses; Figure 3E). At week 26, the reduction in log10 serum HBV DNA was greatest in both groups, with a decrease of 1.49±2.04 in the TAC+ETV group and of 2.47±2.08 in the ETV group in the ITT analyses (P=0.37). In the PP analyses, the decrease in log10 serum HBV DNA from baseline to week 26 was 1.24±2.03 in the TAC+ETV group and 3.00±1.89 in the ETV group (P=0.08).
ITT and PP analysis showed that the change of ALT and AST had no significant difference between the two groups (P>0.05; Figure 3F,3G). In ITT analysis, ALT and AST at week 26 were 24.8±12.5 and 26.8±13.0 U/L in the TAC+ETV group, respectively, while in the ETV group they were 24.2±9.94 and 30.9±9.80 U/L. In PP analysis, ALT and AST at week 26 were 24.5±13.2 and 24.2±11.0 U/L in the TAC+ETV group, and 22.7±10.2 and 29.1±9.69 U/L in the ETV group, respectively.
Safety endpoint
In both ITT and PP analyses, the increase in FBG was significantly greater in the TAC+ETV group at week 6 and week 14 than in the ETV group (P<0.05). At week 6, the increased level of FBG in the TAC+ETV group was 0.82±0.99 mmol/L and was 0.17±0.71 mmol/L in the ETV group in the ITT analyses (P=0.012; Figure 3H). In the PP analyses, the increased level of FBG from baseline to week 6 was 0.85±0.92 mmol/L in the TAC+ETV group and 0.28±0.74 mmol/L in the ETV group (P=0.029). At other follow-up time points, there was no significant difference in the increased level of FBG between the two groups (P>0.05). None of patients (n=11) who had achieved HBV DNA negative conversion had HBV DNA virologic breakthrough in the TAC+ETV group compared to 4 of 14 patients (28.6%) in the ETV group (P=0.11). The tacrolimus blood concentration ranged from 4.25 to 5.86 ng/mL in the TAC+ETV group (Figure 3I).
Adverse events
None of the patients were diagnosed with AKI or ESRD. However, one patient in the ETV group showed a 50% decline in eGFR at week 26, compared to none in the TAC+ETV group. Elevated FBG was detected in 6 of 14 (42.9%) patients in the TAC+ETV group and 4 of 17 (23.5%) patients in the ETV group (P=0.44), which could be controlled using hypoglycemic drugs or adjusting the dose of tacrolimus. One (5.9%) patient in the ETV group experienced a severe adverse event at week 1 and dropped out of the trial due to severe edema. One (7.1%) patient in the TAC+ETV group had muscle spasms. One patient in the TAC+ETV group reached 2 times the upper limit value (2ULN) of ALT and one in the ETV group reached 2ULN of AST. One patient in the ETV group reached 2ULN of ALT and AST in the same follow-up. All patient ALT and AST levels returned to normal at week 26.
Discussion
HBV-GN is one of the most common extrahepatic manifestations of HBV infection and one of the most common secondary causes of glomerulonephritis in China. Nucleos(t)ide is recommended for the treatment of HBV-GN due to favorable suppression of viral replication and improvement of proteinuria (16). However, administration of immunosuppressive agents for the treatment for HBV-GN remains controversial, as while corticosteroids can improve proteinuria, it can also potentially cause reactivation of HBV replication, as well as liver and kidney function damage (17). The combination therapy of immunosuppressive agents and antiviral agents can decrease the rate of HBV reactivation compared to monotherapy with immunosuppressive agents (15). Sodium taurocholate cotransporting polypeptide (NTCP), a multiple transmembrane transporter expressed in the liver, is a functional receptor that interacts with the pre-S1 region of the HBV large envelope protein involved in HBV infection (18). Tacrolimus is effective for treating primary nephropathy and can suppress HBV infection through reducing the binding of HBV to NTCP (12). Xiong et al. reported that tacrolimus therapy for HBV-GN could achieve complete remission (19). Zheng et al. found that antiviral and immunosuppressant combination therapy for HBV-GN improved proteinuria with stable HBV DNA titer and renal function (20).
Therefore, to determine whether tacrolimus and entecavir combination therapy is effective and safe for HBV-GN, a prospective study including 33 HBV-GN patients was conducted. The results demonstrated that tacrolimus and entecavir combination therapy significantly improved sALB levels compared to entecavir monotherapy in HBV-GN patients, with favorable proteinuria remission rates, steady descent of HBV DNA titer, low HBV activation rates, stable renal function, and mild adverse events.
In our study, 9 of 14 (64.3%) patients treated with TAC and ETV combination therapy achieved CR or PR at week 26, compared to 10 of 17 (58.8%) patients treated with ETV monotherapy (P=0.38). The rate of proteinuria remission was lower than that reported in the study by Yan et al., in which 83.7% patients treated telbivudine achieved CR+PR (21). Similar to the latter study, Sun et al. also demonstrated that 85% of patients achieved CR+PR with lamivudine (LAM) therapy at 12 months (22). Lower proteinuria baseline, higher serum albumin baseline, and distinct nucleos(t)ide analogue agents used in the study by Sun et al. might contributed to the different results. However, the proteinuria remission rate in the TAC+ETV group was also lower than that observed in our previous retrospective study showing that 87% of patients treated with TAC and ETV achieved CR+PR and 42% of patients treated with ETV achieved CR+PR at 24 weeks (15). This might be attributed to the higher baseline level of proteinuria and serum HBV DNA titer and the lower baseline level of eGFR in the patients treated with TAC and ETV in this study.
In both ITT and PP analyses, 24 h UP decreased from baseline in both groups, though the decreased level was no statistical difference between the two groups. This may be due to the small sample size of this study, and the improvement of urinary protein in HBV-GN patients with massive proteinuria treated by TAC+ETV needs to be further explored in future studies with larger sample size.
Accompanied with proteinuria remission, our data demonstrated that the extent of increase in serum ALB was also remarkable in both groups, especially in the group given TAC and ETV combination therapy. These results suggest that TAC+ETV may improve the serum albumin level of HBV-GN patients with proteinuria better than entecavir alone. The increasing trend of serum ALB in patients treated with ETV was consistent with other studies (23,24).
The current study found no significant difference in the change in eGFR between the two groups during follow-up, although the eGFR was higher in the ETV group at week 6 and week 10 compared to the TAC+ETV group (P<0.05). However, there was no significant difference in eGFR between the two groups during other follow-up periods (P>0.05). This suggests that TAC+ETV can maintain stable renal function in the treatment of HBV-GN. Our results were similar to other studies showing that ETV could neither improve renal function nor damage renal function (25,26).
This investigation demonstrated that both the ETV group and ETV+TAC group experienced a more significant reduction in HBV DNA titer than patients treated with corticosteroids or lamivudine and mycophenolate mofetil combination therapy in other studies (20,27). The difference may be attributed to the fact that corticosteroids were not used in our study, as corticosteroids might reactivate HBV DNA replication. Furthermore, the antiviral effects of lamivudine may neutralize the latent HBV DNA reactivation. In addition, the type of nucleos(t)ide analogue and immunosuppressive agents in the studies mentioned above were different from the current study (22,27). In addition, none of the patients who accepted TAC and ETV combination therapy had HBV DNA virologic breakthrough compared to 28.6% (4 of 14) of patients in the ETV group (P=0.11). However, there was no statistical difference in the HBV DNA virologic breakthrough between the two groups. This differed from the study by Tsai et al. demonstrating a 3.4% virologic breakthrough rate in the ETV treatment group for chronic hepatitis B patients after 7 years (25). In line with the reduced HBV DNA titer, the levels of ALT and AST also gradually decreased in both groups. Therefore, combination treatment with TAC and ETV did not increase the risk of HBV reactivation, and indeed, TAC may suppress HBV replication by inhibiting the combination of NTCP and HBV.
Only one patient in the ETV group reported severe edema at week 1, which was considered a severe adverse event and this patient dropped out of the study. None of the patients were diagnosed with ESRD or AKI. However, 42.9% of patients in the TAC+ETV group and 23.5% in the ETV group had elevated FBG (P=0.44), which was consistent with TAC elevating blood glucose levels. Although the rise of FBG was transient, it highlights the importance of monitoring FBG in such patients. The elevation in ALT and AST levels over 2ULN were also transient. Most of the adverse events were tolerable and controllable.
There were some limitations to this study. First, the study included a relatively small sample size. The prevalence of HBV-GN is low and patients were excluded for a variety of reasons, including failure of urine protein standards, the use of glucocorticoids and other immunosuppressants, an eGFR of less than 30 mL/min/1.73 m2, and a history of diabetes, among other reasons. Second, it was a single-blinded study and assessors were aware of the treatment assignments which may have caused partial bias in the results. Finally, this investigation was limited by a relatively short follow-up time. As a result, larger sample sizes and longer follow-up periods are needed in future double-blind trials to confirm the efficacy and safety of tacrolimus and entecavir combination therapy in the treatment of patients with HBV-GN.
Conclusions
In adult HBV-GN patients, tacrolimus and entecavir combination therapy may significantly improve serum albumin level without increasing the risk of HBV reactivation, and maintain stable liver and kidney function, compared with entecavir monotherapy.
Acknowledgments
Funding: This study was supported by the Natural Science Foundation of Guangdong Province (Grant No. 2020A1515010137), the Scientific Research Project of Guangdong Provincial People’s Hospital Summit Plan (Grant Nos. KJ012019436; DFJH201911), and the Guangzhou Scientific Research Project (No. 201707010321).
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-328/rc
Trial Protocol: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-328/tp
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-328/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-328/coif). All authors report that this study was supported by the Natural Science Foundation of Guangdong Province (Grant No. 2020A1515010137), the Scientific Research Project of Guangdong Provincial People’s Hospital Summit Plan (Grant Nos. KJ012019436; DFJH201911), and the Guangzhou Scientific Research Project (No. 201707010321). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Guangdong Provincial People’s Hospital Research Ethics Committee [No. GDREC2015287H(R1)] and informed consent was obtained from all patients and the study was approved by institutional review board at all participating institutions
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organization. (2017). Global hepatitis report, 2017. World Health Organization. Available online: http://www.who.int/iris/handle/10665/255016
- Schweitzer A, Horn J, Mikolajczyk RT, et al. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet 2015;386:1546-55. [Crossref] [PubMed]
- Liu J, Liang W, Jing W, et al. Countdown to 2030: eliminating hepatitis B disease, China. Bull World Health Organ 2019;97:230-8. [Crossref] [PubMed]
- Liu J, Zhang S, Wang Q, et al. Seroepidemiology of hepatitis B virus infection in 2 million men aged 21-49 years in rural China: a population-based, cross-sectional study. Lancet Infect Dis 2016;16:80-6. [Crossref] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int 2021;100:S1-S276. [Crossref]
- Lai KN, Li PK, Lui SF, et al. Membranous nephropathy related to hepatitis B virus in adults. N Engl J Med 1991;324:1457-63. [Crossref] [PubMed]
- Ozdamar SO, Gucer S, Tinaztepe K. Hepatitis-B virus associated nephropathies: a clinicopathological study in 14 children. Pediatr Nephrol 2003;18:23-8. [Crossref] [PubMed]
- Yang Y, Ma L, Wang C, et al. Effectiveness of sulodexide might be associated with inhibition of complement system in hepatitis B virus-associated membranous nephropathy: An inspiration from a pilot trial. Eur J Intern Med 2016;32:96-104. [Crossref] [PubMed]
- Perrillo RP, Gish R, Falck-Ytter YT. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology 2015;148:221-44.e3. [Crossref] [PubMed]
- Lai KN, Tam JS, Lin HJ, et al. The therapeutic dilemma of the usage of corticosteroid in patients with membranous nephropathy and persistent hepatitis B virus surface antigenaemia. Nephron 1990;54:12-7. [Crossref] [PubMed]
- Tur-Kaspa R, Burk RD, Shaul Y, et al. Hepatitis B virus DNA contains a glucocorticoid-responsive element. Proc Natl Acad Sci U S A 1986;83:1627-31. [Crossref] [PubMed]
- Watashi K, Sluder A, Daito T, et al. Cyclosporin A and its analogs inhibit hepatitis B virus entry into cultured hepatocytes through targeting a membrane transporter, sodium taurocholate cotransporting polypeptide (NTCP). Hepatology 2014;59:1726-37. [Crossref] [PubMed]
- Nkongolo S, Ni Y, Lempp FA, et al. Cyclosporin A inhibits hepatitis B and hepatitis D virus entry by cyclophilin-independent interference with the NTCP receptor. J Hepatol 2014;60:723-31. [Crossref] [PubMed]
- Liao R, Liu Q, Zheng Z, et al. Tacrolimus Protects Podocytes from Injury in Lupus Nephritis Partly by Stabilizing the Cytoskeleton and Inhibiting Podocyte Apoptosis. PLoS One 2015;10:e0132724. [Crossref] [PubMed]
- Wang L, Ye Z, Liang H, et al. The combination of tacrolimus and entecavir improves the remission of HBV-associated glomerulonephritis without enhancing viral replication. Am J Transl Res 2016;8:1593-600. [Crossref] [PubMed]
- Fu B, Ji Y, Hu S, et al. Efficacy and safety of anti-viral therapy for Hepatitis B virus-associated glomerulonephritis: A meta-analysis. PLoS One 2020;15:e0227532. [Crossref] [PubMed]
- Fang J, Li W, Tan M, et al. Discontinuation of antiviral prophylaxis increased the risk of hepatitis B virus reactivation in glomerulonephritis patients under immunotherapy: a real-life observation. Int Urol Nephrol 2018;50:1653-60. [Crossref] [PubMed]
- Yan H, Zhong G, Xu G, et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife 2012;1:e00049. [Crossref] [PubMed]
- Xiong QF, Zhong YD, Hu ZL, et al. Successful treatment of occult hepatitis B virus infection related membranous nephropathy after entecavir therapy. Acta Clin Belg 2015;70:223-5. [Crossref] [PubMed]
- Zheng XY, Wei RB, Tang L, et al. Meta-analysis of combined therapy for adult hepatitis B virus-associated glomerulonephritis. World J Gastroenterol 2012;18:821-32. [Crossref] [PubMed]
- Yan Z, Qiao B, Zhang H, et al. Effectiveness of telbivudine antiviral treatment in patients with hepatitis B virus-associated glomerulonephritis: A 104-week pilot study. Medicine (Baltimore) 2018;97:e11716. [Crossref] [PubMed]
- Sun LJ, Shan JP, Cui RL, et al. Combination therapy with lamivudine and angiotensin-converting enzyme inhibitor/angiotensin receptor blocker for hepatitis B virus-associated glomerulonephritis with mild to moderate proteinuria: a clinical review of 38 cases. Int Urol Nephrol 2017;49:1049-56. [Crossref] [PubMed]
- Kataoka H, Mochizuki T, Akihisa T, et al. Successful entecavir plus prednisolone treatment for hepatitis B virus-associated membranoproliferative glomerulonephritis: A case report. Medicine (Baltimore) 2019;98:e14014. [Crossref] [PubMed]
- Yang YF, Xiong QF, Zhao W, et al. Complete remission of hepatitis B virus-related membranous nephropathy after entecavir monotherapy. Clin Res Hepatol Gastroenterol 2012;36:e89-92. [Crossref] [PubMed]
- Tsai MC, Chen CH, Tseng PL, et al. Comparison of renal safety and efficacy of telbivudine, entecavir and tenofovir treatment in chronic hepatitis B patients: real world experience. Clin Microbiol Infect 2016;22:95.e1-7. [Crossref] [PubMed]
- Tsai HJ, Chuang YW, Lee SW, et al. Using the chronic kidney disease guidelines to evaluate the renal safety of tenofovir disoproxil fumarate in hepatitis B patients. Aliment Pharmacol Ther 2018;47:1673-81. [Crossref] [PubMed]
- Wu SB, Wang YD, Xu Y. Study of mycophenolate mofetil and lamivudine in treatment of hepatitis B virus associated glomerulonephritis. Xiandai Zhongxiyi Jiehe Zazhi 2008;17:4674-4676.


