
Expert consensus on multi-disciplinary treatment, whole-course pulmonary rehabilitation management in patients with lung cancer and chronic obstructive lung disease
Introduction
Lung cancer, a malignant tumor originating from respiratory epithelial cells in bronchi, bronchioles, or alveoli, is the leading cause of cancer death in China (1). Chronic obstructive pulmonary disease (COPD) is characterized by persistent respiratory symptoms and airflow limitation due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious particles or gases, and is currently the fifth leading cause of death in China (2). Research has shown that COPD and lung cancer share common risk factors and pathogenic mechanisms including smoking, inflammatory pathways, oxidative stress, and pulmonary dysbiosis (3-6). Clinically, 40% to 70% of lung cancer patients suffer from COPD, whereas COPD patients also have a high incidence of lung cancer, with an annual incidence rate of about 16.7% (7,8).
While surgery is the mainstay of treatment for early- and locally-advanced lung cancer (9), patients often have reduced lung function after lung surgery. It was reported that the vital capacity and maximal voluntary ventilation (MVV) decreased by, respectively, 20–33.5% and 13–26% after pneumonectomy, 16–27% and 13.6% after lobectomy, and by 11.2% and 11.6% after segmentectomy (10). This may be due to the destruction of the integrity of the thoracic cage on the surgical side, damage to the intercostal muscles and intercostal nerves during surgery, pleural adhesions, postoperative wound pain, excessively tight dressings, and pleural effusion and gas (11-13). COPD significantly increases the incidence of postoperative complications of lung cancer and affects postoperative anticancer therapy. Poorer lung function is closely associated with longer perioperative antibiotic use, longer duration of mechanical ventilation, and a higher incidence of pneumonia (14). Therefore, pulmonary rehabilitation (PR) is recommended for patients with COPD undergoing lung cancer surgery, with an attempt to reduce surgical complications and promote postoperative respiratory function recovery.
According to the “2015 An Official American Thoracic Society/European Respiratory Society Policy Statement: Enhancing Implementation, Use, and Delivery of Respiratory Rehabilitation”, PR is “a comprehensive intervention based on a thorough patient assessment followed by patient-tailored therapies that include, but are not limited to, exercise training, education, and behavior change, designed to improve the physical and psychological condition of people with chronic respiratory disease and to promote the long-term adherence to health-enhancing behaviors (15,16).” A well-designed PR program should comprise exercise training, drug therapy, smoking cessation, nutritional support, behavioral changes, and health education.
Therefore, in order to standardize the PR programs for patients with both lung cancer and COPD who undergo lung cancer surgery, the Lung Cancer Professional Committee of China Medicine Education Association convened top experts in respiratory medicine, medical oncology, thoracic surgery, radiotherapy, and nursing to explore this topic. After discussion and revisions, the final expert consensus statements were formed as follows.
Methods
The recommended users of this expert consensus are doctors and nurses related to clinical diagnosis and treatment of lung cancer, and the target population is patients with lung cancer comorbidity of COPD and receiving surgery operation. Respiratory physician, thoracic surgeons, lung oncologists, nursing staff, etc., participate in making this expert consensus.
The process of expert consensus strictly follows the WHO guidelines. All members participating in this expert consensus have no conflict of interest related to this consensus.
This study adopts the method of consensus meeting. According to the requirements and methods of evidence-based medicine, the working group systematically searched the relevant literature on this topic. Four English literature databases were searched systematically: PubMed, Cochrane Library, EMBASE and web of science. The working group screened out the relevant evidence in the literature, formed the first draft of the evidence, formed a questionnaire on low-level evidence and submitted it to the consensus meeting. More than 20 experts were selected to participate in the consensus meeting. The first draft was discussed freely and low-level evidence was voted to form a preliminary expert consensus. In the preliminary expert consensus, the experts who did not participate i were invited again for external verification, and the final draft was formed.
Respiratory assessment
Respiratory assessment includes history taking, respiratory symptom assessment, physical examination, laboratory tests, instrumental examinations (including respiratory function examination), exercise ability assessment, nutritional assessment, social function assessment, mental assessment, and COPD assessment (Table 1). It may be patient-tailored.
Table 1
| Category | Item |
|---|---|
| Name | |
| Date of assessment | |
| Time of assessment | Before surgery/months after surgery |
| Previous disease | |
| Surgery | |
| Medications | |
| Medical histories | |
| Respiratory symptoms | Dyspnea |
| mMRC | |
| Borg scale | |
| Visual Analogue Scale | |
| fatigue | |
| FSS | |
| ICFS | |
| Physical examination | Respiratory rate |
| Heart rate | |
| Pulse | |
| Body temperature | |
| SpO2 | |
| Pain score | |
| Body height | |
| Body weight | |
| Physical examination of the respiratory system | |
| Physical examination of the cardiovascular system | |
| Limbs | |
| Laboratory tests | Routine blood test |
| Biochemistry | |
| Blood gas analysis | |
| Instrumental examinations (including respiratory function examination) | Electrocardiogram |
| Echocardiography | |
| Bone mineral density | |
| Lung function FEV1 | |
| FVC | |
| FEV1/FVC% | |
| DLCO% | |
| PEF | |
| MVV | |
| Maximum expiratory pressure | |
| Maximum inspiratory pressure | |
| Exercise capability | 6MWT |
| ISWT | |
| ESWT | |
| Stair climbing test | |
| Cardiopulmonary exercise test | |
| Nutrition | BMI |
| Fat mass to fat-free mass ratio | |
| Muscle mass | |
| Social function | Activities of daily living (ADL) |
| Smoking status | |
| Disease awareness | |
| Education level | |
| Psychological assessment and counseling | BAI |
| BDI-II | |
| PHQ-9 | |
| HADS | |
| COPD assessment | BODE index |
| Comprehensive assessment of the severity of stable COPD (A/B/C/D) |
BODE, BMI, obstructive, dyspnea, endurance; 6MWT, 6-minute walk test; BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory; BMI, body mass index; Borg scale, Borg Dyspnea Scale; COPD, chronic obstructive pulmonary disease; DLCO%, diffusing capacity of the lungs for carbon monoxide; ESWT, endurance shuttle walk test; FEV1, forced expiratory volume in one second; FSS, Fatigue Severity Scale; FVC, forced vital capacity; HADS, Hospital Anxiety and Depression Scale; ICFS, Identity-Consequence Fatigue Scale; ISWT, incremental shuttle walk test; mMRC, modified British Medical Research Council Scale; MVV, maximal voluntary ventilation; PEF, peak expiratory flow; PHQ-9, Patient Health Questionnaire-9; SpO2, peripheral capillary oxygen saturation.
Timing of pulmonary assessment for PR
Preoperative assessment
If a lung cancer patient scheduled for a surgery is known to have a history of COPD, a thorough pulmonary assessment shall be performed before the lung cancer surgery. PR will be offered according to the respiratory status, and a second assessment shall be performed upon the completion of the PR program. If COPD is found during the routine preoperative evaluation of a patient with lung cancer, other pulmonary assessment items shall be performed on top of routine preoperative examinations. PR is then offered according to the respiratory status, and a second assessment for PR will be performed upon the completion of preoperative PR.
Postoperative assessment
Patients who have received PR after surgery are re-assessed for their respiratory status at 1 month, 3 months, 6 months, and 1 year postoperatively, and the PR strategy shall be adjusted according to the assessment results. Pulmonary function testing is not recommended within 1 month after surgery as it may lead to rupture of the surgical incision.
Elements of respiratory assessment
History taking
For patients with lung cancer complicated by COPD who are scheduled to receive a surgery for lung cancer, face-to-face communication is required before starting the PR program to inform the patients and their families about the value, items, and precautions of the PR program. During the communication, the patients will be inquired about their medical histories, medications, and surgical history. Notably, for patients with occult unstable angina pectoris, PR should not be started before the condition is stabilized. In addition, the exercise prescriptions need to be tailored for patients with concomitant neurological and/or musculoskeletal diseases.
Assessment of respiratory symptoms: the common symptoms in patients with lung cancer and COPD include dyspnea and fatigue
- Dyspnea: dyspnea is a main symptom of COPD, and lung cancer patients may also experience dyspnea due to the obstruction of the bronchial lumen by a mass or cancerous pleural effusion. Dyspnea can be assessed by using a variety of scales including the modified British Medical Research Council (mMRC) Dyspnea Scale, the Borg Dyspnea Scale, and the Visual Analogue Scale (VAS) for dyspnea. The mMRC scale is the most commonly used scale. It can be divided into 5 grades, and a higher grade indicates more severe dyspnea. The Borg Dyspnea Scale rates from 0 to 10, with 0 at nothing and 10 at intense exertion. It is often used to assess the respiratory status of patients before and after the 6-minute walk test (6MWT). The VAS is composed of a 10-cm horizontal or vertical line. The patients are asked to mark their current dyspnea level on the line. The distance between the left (or top) end to the marked site is measured to yield a dyspnea score, and a higher score means more severe dyspnea.
- Fatigue: lung cancer patients with COPD often feel fatigue, which can be caused by the release of various cytokines by the tumor and/or as a side effect of anticancer treatment. In addition, shortness of breath, muscle atrophy, anemia, and many other COPD-related conditions can also aggravate fatigue. Fatigue is often assessed by using the Fatigue Severity Scale (FSS), which consists of 9 items with a 7-point Likert scale. A higher FSS score indicates more severe fatigue. In addition, perioperative fatigue can be assessed by using the Identity-Consequence Fatigue Scale (ICFS) and other scales.
Physical examination
- Vital signs: including respiratory rate, blood pressure, pulse, body temperature, pain, and peripheral capillary oxygen saturation (SpO2);
- Physical examination: including height and weight;
- Respiratory system: including patterns of respiratory muscle use, respiratory movement, diaphragm movement, and abnormal breath sounds;
- Cardiovascular system: including heart rate, heart rhythm, heart murmurs, extra heart sounds, and peripheral vascular signs; and
- Limbs: including joint mobility, clubbing of the fingers/toes, and muscle atrophy.
Laboratory tests
Including routine blood and biochemical tests (liver function, renal function, electrolytes, and blood sugar, among others); peripheral arterial blood gas (ABG) analysis may be performed if necessary.
Instrumental examinations (including respiratory function test)
Including electrocardiography and/or dynamic electrocardiography, cardiac ultrasound, pulmonary artery pressure measurement, bone mineral density test, and pulmonary function test. A thorough preoperative pulmonary function test shall include spirometry, static lung volumes, and diffusing capacity. The relevant indicators should include forced expiratory volume in one second (FEV1) and forced vital capacity (FVC). Some of the postoperative pulmonary functions can be predicted based on the number of resected lung segments by using the Johl and Frost formula (17). Cardiopulmonary exercise testing (CPET) may be optional if the condition allows. The measurement of maximum expiratory pressure (MEP) and maximum inspiratory pressure (MIP) may refer to the “Chinese Guidelines on Pulmonary Function Testing” developed by the Pulmonary Function Study Group of the Chinese Thoracic Society.
Exercise ability assessment
The 6MWT, shuttle walking test (SWT), stair-climbing test (SCT), and CPET are recommended to assess patients’ exercise ability.
- The 6MWT: this test assesses cardiorespiratory status by measuring the distance a patient walks at the fastest pace for 6 minutes. The test is simple to perform and can accurately reflect a patient’s walking capacity;
- The SWT: this test includes the incremental shuttle walking test (ISWT) and endurance shuttle walking test (ESWT);
- The SCT: if a patient can quickly climb 5 floors by ascending 2 stair steps per stride, his/her cardiopulmonary function is good; if a patient can climb 5 floors by ascending 1 step per stride without obviously deep and fast breaths, it means their cardiopulmonary function is fair; if a patient ascends 5 floors step by step, with wheezing and shortness of breath, it indicates that the adaptability of their cardiopulmonary function is poor; if the patient ascends the 3rd floor, the patient experiences shortness of breath, indicating that their cardiopulmonary function is very poor;
- CPET: it is often important to measure the oxygen uptake (VO2), peak oxygen uptake (peak VO2), and peak oxygen uptake as predicted (peak VO2%) during exercise, upon the completion of exercise, and during the recovery period.
Nutritional assessment
Including height, weight, body mass index (BMI), fat mass to fat-free mass ratio, and muscle mass.
Assessment of social function
- Activities of daily living (ADL) assessments: including basic daily living activities (changing clothes and bathing, among others), housework, leisure activities, occupation-related activities, and sexual behavior;
- Assessment of smoking status: including past and current smoking status and whether there is tobacco addiction or dependence;
- Disease awareness: whether the patient can correctly recognize the current disease status determines whether the patient can follow the doctor’s orders;
- Educational level: the educational level of a patient is mainly measured by the highest academic degree obtained by the patient, which determines whether the patient can understand the instructions given by the medical staff and whether he/she can cooperate with the medical staff to complete the corresponding treatment items.
Assessment of mental status
- Anxiety: the Beck Anxiety Inventory (BAI) is recommended;
- Depression: the Beck Depression Inventory-II (BDI-II), Patient Health Questionnaire-9 (PHQ-9), and other scales are recommended;
- Anxiety and depression: scales such as the Hospital Anxiety and Depression Scale (HADS) can be used to screen for both anxiety and depression simultaneously.
Assessment of COPD
The BODE index (BMI, obstructive, dyspnea, endurance), which is based on BMI, airway obstruction (FEV1), dyspnea scale (mMRC), and exercise capacity (6WMT), is recommended. In addition, the comprehensive assessment of the severity of stable COPD (A/B/C/D group) system, which includes spirometry results, history of COPD exacerbations, mMRC, and the COPD assessment test (CAT), is also useful in the assessment of COPD.
Timing of PR
Patients are recommended to undergo preoperative PR and postoperative long-term PR.
Preoperative PR can help improve cardiopulmonary function, reduce surgical complications, shorten postoperative hospital stay, and lower medical expenses. Patients are recommended to undergo PR for about 2 weeks (5 days per week) before surgery to achieve good effects.
Postoperative PR ameliorates dyspnea, exercise intolerance, and wound pain due to surgery and improves anxiety/depression caused by the disease itself. Short- or long-term PR has positive effects on improving lung function and quality of life. Postoperative PR usually lasts 1–2 months, and a 20-week program has also been described in the literature. The frequency of PR is typically 3–5 times per week.
PR strategies
Exercises
Exercise includes endurance training, interval training, resistance/strength training, upper limb training, flexibility training, inspiratory muscle training, and neuromuscular electrical stimulation.
All exercise prescriptions shall follow the principles of frequency, intensity, time, and type (FITT) (18,19). The American College of Sports Medicine (ACSM) recommends the following FITT principles for COPD patients:
- Frequency: at least 3–5 days a week;
- Intensity: moderate to high intensity [i.e., at 50–80% of predetermined peak power output, or 4–6 points on the Borg CR-10 scale, or 12–14 points on the Borg rating of perceived exertion (RPE)];
- Time: 20–60 minutes of moderate-to-high-intensity exercise a day; if it cannot be completed, intersperse several short periods of low-intensity intermittent exercise or rest in the 20-minute (or longer) exercise; and
- Type: it is usually an aerobic exercise, including walking (walking outdoors or walking on a treadmill), stationary bike, and upper body hand bike.
Exercise training includes the following types:
- Endurance training: high-intensity endurance training is frequently performed in a PR program (20), often based on cycling or walking exercises (21-23). It helps to adjust walking muscles, improve cardiopulmonary function, increase physical activity, and ameliorate dyspnea and fatigue. Low-intensity endurance training or interval training is recommended for patients with poor performance status (PS) who have difficulty reaching target intensities or training time (24,25). According to the current conditions in China, stair climbing is also recommended for endurance training;
- Interval training: during interval training, the high-intensity exercise is regularly interspersed by short periods of rest or low-intensity exercise. There is evidence that interval training is associated with lower symptom scores while achieving higher training intensity in patients with COPD (25,26);
- Resistance/strength training: resistance/strength training is the training of local muscle groups through repetitive heavy-load lifting (27,28), whereas resistance training has greater potential to improve muscle mass and strength. In addition to the expected effects on muscle strength, resistance training may also help maintain or improve bone density (21). Furthermore, strength training reduces dyspnea during exercise, making it more acceptable to patients (29);
- Upper limb training: many ADLs including changing clothes, bathing, shopping, and doing housework involve the upper limbs (30). Upper limb training, including aerobic and resistance training, can increase the strength of upper limbs (31). The targeted muscle groups include biceps, triceps, deltoids, latissimus dorsi, and pectoralis;
- Flexibility training: due to the coupling between breathing and posture, abnormal posture is associated with decreased lung function, lower quality of life, reduced bone mineral density, and increased work of breathing (32,33). Improving chest mobility and posture can increase chronic lung capacity in patients with chronic respiratory disease (34). It is recommended that patients perform upper and lower body flexibility exercises at least 2–3 days per week, including the stretching of major muscle groups such as the calves, hamstrings, quadriceps, and biceps and range-of-motion exercises for the neck, shoulders, and trunk;
- Inspiratory muscle training: due to lung hyperdistention in patients with COPD before lung cancer surgery, factors such as muscle/nerve injury caused by the surgery and pain in the surgical incision can lead to respiratory muscle insufficiency. Inspiratory muscle training in patients with weakened inspiratory muscles can improve the exercise capacity and ameliorate dyspnea. The most commonly used form of inspiratory muscle training is resistance training, also called inspiratory pressure threshold loading (IPTL) (35). Inspiratory muscle strength and endurance can be improved when patients perform inspiratory muscle training at a load equal to or greater than 30% of their MIP (36,37);
- Neuromuscular electrical stimulation: transcutaneous neuromuscular electrical stimulation of skeletal muscle is an alternative rehabilitation technique that induces muscle contractions to train specific muscles without the need for routine exercise. Specific stimulation intensity (amplitude), frequency, duration, and waveform are selected during manipulation to achieve the desired muscle response (33,38). Muscle contractions induced by electrical stimulation have minimal cardiac demands, do not cause dyspnea, and can avoid the adverse cognitive and psychological factors that patients may experience during routine exercise (39,40). Therefore, neuromuscular electrical stimulation is particularly useful in patients with severe ventilation and/or cardiac dysfunction.
Medical treatment
Bronchodilators
Bronchodilators play a key role in COPD management. They can control symptoms, reduce deterioration in lung function, and improve exercise tolerance and health status (41). Inhaled bronchodilators, which act primarily on airway smooth muscle, not only improve expiratory flow in patients with airflow limitation but also ameliorate resting and dynamic lung hyperdistention (41,42). Both short- and long-acting bronchodilators can increase exercise capacity in patients with COPD (42,43). Under optimal bronchodilator therapy, the main exercise limitation changes from dyspnea to leg fatigue, allowing the patient to exercise the surrounding muscles to a greater extent. This confirms a potential synergy between medical and non-medical treatments. For lung cancer patients with COPD, adjusting and optimizing bronchodilator therapy before exercise training is a routine strategy for PR. According to the Global Initiative Chronic Obstructive Lung Disease (GOLD) guidelines, patients with stable COPD may be classified into 4 groups, with different treatment options, namely group A: a bronchodilator; group B: a long-acting bronchodilator (long-acting anticholinergic agent or long-acting beta2 agonist); group C: a long-acting anticholinergic agent; and group D: a long-acting anticholinergic agent, or a long-acting anticholinergic agent in combination with a long-acting beta2 agonist, or an inhaled corticosteroid in combination with a long-acting beta2 agonist (41). Most medications for COPD are administered through a nebulizer. It is necessary to select appropriate drugs and instruct patients to use them correctly.
Anabolic hormones
In theory, these drugs can enhance muscle strength (by inducing muscle fiber hypertrophy) or endurance (by increasing capillary density, mitochondrial count, and oxidative metabolic enzyme concentration), thereby enhancing the effects of strength training or endurance training, respectively. The use of testosterone analogs may result in muscle mass gain, though this lacks consistent evidence (44-46). Two small studies in COPD patients demonstrated that growth hormone increased weight, but there was no evidence of improvement in peripheral muscular endurance or strength (47,48).
Respiratory therapy
Before the initiation of a PR program, ABG and SpO2 should be assessed and recorded as the baseline values. The volume of inspired oxygen shall be adjusted based on changes in SpO2 values and ABG results (e.g., in patients with hypercapnia). Oxygen titration can ensure a SpO2 of above 90%, ideally 92%.
Oxygen therapy
For patients with hypoxemia at rest, long-term home oxygen therapy is recommended, which has beneficial effects on their hemodynamics, respiratory physiology, exercise tolerance, and mental state and can improve quality of life and increase survival rates. Long-term home oxygen therapy is indicated if one of the following criteria is met: (I) resting arterial oxygen partial pressure (PaO2) is ≤55% or oxygen saturation (SaO2) <88%, with or without hypercapnia; or (II) PaO2 56–60 mmHg and/or SaO2 <89%, with one of the following conditions: secondary polycythemia (hematocrit >55%); pulmonary hypertension (mean pulmonary arterial pressure ≥25 mmHg); and/or edema due to right-sided cardiac dysfunction. Oxygen therapy is usually delivered via nasal cannula, with appropriate oxygen flow rates of 1–2 L/min. Oxygen inhalation typically lasts more than 15 hours per day to ensure the resting PaO2 reaches ≥60 mmHg and/or the resting SaO2 increases to 90% or higher (2).
Noninvasive ventilation
Noninvasive positive pressure ventilation (NPPV) can help COPD patients relax their respiratory muscles and reduce the work of breathing during breathing exercises. NPPV is associated with decreased symptoms of dyspnea, improved gas exchange, increased minute ventilation, and increased exercise time. Therefore, NPPV can be used as an adjuvant therapy for PR (49-52), and 2 noninvasive ventilation strategies are usually used: (I) NPPV during exercise, including continuous positive airway pressure (CPAP), pressure-support ventilation, and proportional assist ventilation (PAV); and (II) nocturnal NPPV during non-training intervals.
Airway secretion clearance
- Effective cough: instruct patients to perform effective cough, which can voluntarily clear airway secretions;
- Postural drainage and expectoration: the body is placed in different positions based on the anatomy of the bronchial tree to drain the lung contents and sputum to the large trachea utilizing the action of gravity, thus allowing the discharge of the secretions together with correct breathing and expectoration methods;
- Mechanical vibration sputum expectoration: the chest wall is mechanically vibrated, and accordingly the airway is vibrated to make the secretions on the airway fall off.
Nutritional support
Patients with COPD and lung cancer often have changes in nutritional status. Research has shown that 20–30% of normal-weight COPD patients have muscle wasting and relative fat gain (53), and weight loss and being underweight are most common in patients with advanced lung cancer (54). Weight loss and underweight status are associated with increased mortality in patients with either lung cancer or COPD, and several studies on improving body composition abnormalities in such patients have yielded positive results (55-57). BMI is a simple indicator for nutritional assessment. Active nutritional intervention is required for patients with BMI <25 kg/m2. Initial nutritional therapy involves the adjustment of dietary habits and food types, which can be combined with exercise training. If the initial nutritional therapy is ineffective, caloric supplementation can be further increased, even by the use of enteral nutrition.
Health education and psychological counseling/therapy
- Smoking cessation: smoking is an independent risk factor for pulmonary complications after lung resection (58) and has an adverse effect on the long-term survival of lung cancer patients after lung resection (59). Smoking cessation before surgery reduces the incidence of postoperative pulmonary complications (60) and also significantly increases the survival rate of lung cancer patients (61). Therefore, smoking status should be determined as early as possible in the diagnosis and treatment of lung cancer, and smoking cessation advice and support should be provided to smokers. Patients are generally required to quit smoking strictly for 2 weeks before surgery or for life;
- Psychological counseling and therapy: including behavior change (62,63), cognitive adjustment (64), self-efficacy improvement (65), and collaborative self-management (66);
- Social function: an ideal PR program should also involve the patient’s spouse or other caregivers. Physicians shall communicate the importance of PR to the patient’s spouse or caregiver so that they can supervise the patient’s adherence to doctor’s orders and provide necessary medical care during PR.
Perioperative PR management strategies in lung cancer patients with COPD
Figure 1 summarizes the perioperative PR management.
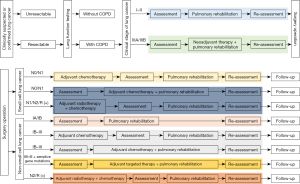
Preoperative PR
The effects of preoperative PR in patients with lung cancer complicated by COPD have been widely explored (Table 2) (67-81), mainly including improving lung function, enhancing cardiopulmonary exercise function, increasing exercise tolerance, and reducing the average hospital stay. Preoperative rehabilitation usually adopts a 2-week PR program including exercise, smoking cessation, and respiratory therapy.
Table 2
| Authors | Year | Time | Time frequency | Pulmonary rehabilitation strategies | Main outcome measures | References |
|---|---|---|---|---|---|---|
| Bobbio A, et al. | 2008 | 4 weeks | 5 sessions/week | Bronchodilators; physical therapy (controlled breathing and coughing techniques); incentive spirometry; and peripheral muscle exercise training | FEV1; CPET (VO2peak) | (67) |
| Divisi D, et al. | 2013 | 4–6 weeks | 6 sessions/week | (I) Breathing exercises (using positive pressure ventilation); (II) inspiratory muscle training; (III) postural drainage; (IV) aerobic exercise (power cycling and walking) | FEV1, FVC, CPET (VO2peak, working load, and ventilation volume) | (68) |
| Coats V, et al. | 2013 | 4 weeks | 3–5 sessions/week | Aerobic training and strength training | 6WMT, HADS, SF-36, EORTC-QLQ-C30, and EROTC-LC13 | (69) |
| Stefanelli F, et al. | 2013 | 3 weeks | 5 sessions/week | High-intensity upper and lower limb muscle training (upper limbs: rowing ergometer; lower limbs: power cycling and stair climbing); breathing training | FEV1, CPET (VO2peak), and Borg scale | (70) |
| Morano MT, et al. | 2013 | 4 weeks | 5 sessions/week | Warm-up; upper and lower extremity stretching exercises; upper body workout; aerobic physical activity; inspiratory muscle training | 6MWT; FEV1; FVC; MEP; MIP | (71) |
| Mujovic N, et al. | 2014 | 2–4 weeks | 5 sessions/week | Nebulized inhalation, thoracic expansion, diaphragmatic breathing, and movement of shoulder girdle | FEV1, FVC, 6MWT, Brog scale | (72) |
| Morano MT, et al. | 2014 | 4 weeks | 5 sessions/week | Incremental UULEX, lower extremity endurance training (treadmill); inspiratory muscle training; flexibility, stretching, and balance exercises | UULEX, endurance testing, HADS, 6WMT | (73) |
| Tarumi S, et al. | 2015 | 10 weeks | NA | (I) Breathing with muscle relaxation; (II) breathing training including diaphragmatic breathing, expiratory muscle training, and incentive spirometry; (III) cough training; (IV) lower extremity exercise; (V) ADL training; (VI) treatment of COPD; (VII) smoking cessation | FEV1 and FVC | (74) |
| Mujovic N, et al. | 2015 | 2–4 weeks | 5 sessions/week | (I) Intravenous use of bronchodilators; (II) systemic rehabilitation; (III) nebulized inhalation; and (IV) diaphragmatic breathing, chest expansion, and shoulder exercise | Total hospital stay, FEV1, and 6MWT | (75) |
| Vagvolgyi A, et al. | 2017 | 3 weeks | NA | Breathing training, thoracic exercise, breathing control, drug inhalation, expectoration, psychological improvement, and smoking cessation | FEV1, FVC, 6MWT, grip strength, CAT, and mMRC | (76) |
| Hashmi A, et al. | 2017 | NA | 16 sessions in total | Pursed lip breathing, diaphragmatic breathing, airway clearance, health education, and smoking cessation | FEV1 and FVC | (77) |
| Lai YT, et al. | 2017 | 7 days | NA | (I) Abdominal breathing training; (II) exhalation exercise: using breathing training equipment (Voldyne 5000, Sherwood Medical Supplies, St. Louis, MO, USA); (III) aerobic endurance training: using NuStep equipment (NuStep, Inc., Ann Arbor, MI, USA) | Total hospital stay, FEV1, 6MWT, and QoL | (78) |
| Lai YT, et al. | 2017 | 7 days | NA | (I) Chest expansion and incentive spirometry; (II) abdominal breathing; and (III) aerobic endurance training (Nu-Step device) | Total hospital stay, 6MWT, PEF, QoL, and dyspnea score | (79) |
| Vagvolgyi A, et al. | 2018 | 3 weeks | NA | Breathing training, thoracic exercise, breathing control, drug inhalation, expectoration, psychological improvement, and smoking cessation | 6MWT, chest wall movement, breath-holding time, grip strength, CAT, and mMRC | (80) |
| Marhic A, et al. | 2019 | NA | NA | Smoking cessation; health education, psychological support, and nutritional support; lower extremity aerobic training: power cycling; noninvasive ventilation | CPET (VO2peak) | (81) |
ADL, activities of daily living; CPET, cardiopulmonary exercise testing; FEV1, forced expiratory volume in one second; VO2peak, peak oxygen uptake; FVC, forced vital capacity; 6MWT, 6-minute walk test; HADS, Hospital Anxiety and Depression Scale; EORTC-QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30; LC13, Lung Cancer-Specific Questionnaire; Borg scale, Borg Dyspnea Scale; CAT, COPD Assessment Test; COPD, chronic obstructive pulmonary disease; mMRC, modified British Medical Research Council Dyspnea Scale; QoL, quality of life; PEF, peak expiratory flow; UULEX, unsupported upper limb exercise test; MEP, Maximum expiratory pressure; MIP, maximal inspiratory pressure; NA, not available.
- For patients with stage I–II lung cancer accompanied by COPD, surgery can be performed directly. Prior to surgical treatment, the PR program may be conducted and evaluated as scheduled;
- According to the 2021 edition of the guidelines issued by the Chinese Society of Clinical Oncology (CSCO) and the US National Comprehensive Cancer Network (NCCN), positron emission tomography (PET), computed tomography (CT), endobronchial ultrasound (EBUS), endobronchial ultrasonography (EUS), or mediastinoscopy may be applied for lymph node staging in stage IIIA/IIIB (T3N2M0) non-small cell lung cancer (NSCLC) patients with COPD, and those with operable lesions can receive neoadjuvant therapy. Neoadjuvant therapy includes neoadjuvant chemotherapy and neoadjuvant chemoradiotherapy. Complete surgical resection after neoadjuvant concurrent chemoradiotherapy is currently recommended for patients with superior sulcus tumors with local invasion to the chest wall but without mediastinal lymph node metastasis (T3N1). Many randomized controlled studies have investigated various combinations of neoadjuvant therapy and surgical resection, and the main adverse reactions reported included neutropenia, nausea/vomiting, diarrhea, and radiation pneumonitis (82-84). When designing a PR program (especially exercise training), attention should be paid to the toxicities of neoadjuvant chemotherapy and chemotherapy. For example, patients receiving paclitaxel treatment may be at risk of arrhythmia or blood pressure changes, in whom cardiovascular function should be closely monitored during exercise, and a medical referral will be required if discomfort occurs. Chemotherapy often results in bone marrow suppression; therefore, trauma should be avoided during exercise for patients experiencing thrombocytopenia and infection should be prevented in patients suffering from leukopenia. Radiation pneumonitis is a common adverse reaction of radiotherapy. For mild patients who do not need glucocorticoid therapy, radiation pneumonitis does not affect the implementation of PR. However, for severe radiation pneumonitis patients who require glucocorticoid therapy, PR should be suspended immediately, along with glucocorticoid, oxygen, antibiotics, and other symptomatic treatments. After the radiation pneumonia is improved, PR may be resumed after evaluation by health professionals.
Postoperative PR
The role of postoperative PR in patients with lung cancer complicated by COPD has been widely explored (Table 3) (76,80,85-88). Systematic postoperative rehabilitation exercise usually starts from the sixth week after discharge or upon the completion of chemotherapy. Postoperative rehabilitation usually lasts 1–2 months, and a 20-week program has also been described in the literature. Similar to preoperative PR, most postoperative PR programs include exercise training. The improvement after postoperative PR is mainly reflected in lung function, cardiopulmonary exercise function, and physical activity level (PAL). In addition, Quist’s study has also compared the value of early (2 weeks after surgery) versus late (14 weeks after surgery) PR programs and found that the early program could better improve lung function and endurance indicators (89).
Table 3
| Authors | Year | Time | Frequency | Time of pulmonary rehabilitation in total | Pulmonary rehabilitation strategies | Main outcome measures | References |
|---|---|---|---|---|---|---|---|
| Edvardsen E, et al. | 2015 | 4–6 weeks after surgery | 3 sessions/week | 20 weeks | (High-intensity) cardiovascular warm-up, interval training, progressive resistance training, and daily inspiratory muscle training | Tlco, MVV pred%, CPET (VO2peak), SF-36; EORTC-QLQ-C30 Dyspnea | (85) |
| Maeda K, et al. | 2016 | Postoperative discharge (not mentioned) | 2 sessions/week | 8 weeks | Breathing training is performed on a bench, followed by 20-min high-intensity lower extremity training on a treadmill or ergometer | PAL | (86) |
| Cavalheri V, et al. | 2017 | 6–10 weeks after surgery/4–6 weeks after the end of postoperative chemotherapy | 60 minutes/day | 8 weeks | Aerobic exercise (walking/cycling) and resistance exercise (upper/lower extremity) | 6MWT, CPET (VO2peak and maximum work rate), SF-36, FACT-L, and EORTC-QLQ-C30 | (87) |
| Vagvolgyi A, et al. | 2017 | NA | NA | 3 weeks | Breathing training, thoracic exercise, breathing control, drug inhalation, expectoration, psychological improvement, and smoking cessation | FEV1, FVC, 6MWT, grip strength, mMRC, and CAT | (76) |
| Vagvolgyi A, et al. | 2018 | NA | NA | 3 weeks | Breathing training, thoracic exercise, breathing control, drug inhalation, expectoration, psychological improvement, and smoking cessation | FVC pred%, 6MWT, grip strength, chest wall movement, breath holding time, mMRC, and CAT | (80) |
| Messaggi-Sartor M, et al. | 2019 | 6–8 weeks after discharge (excluding patients receiving radiotherapy or chemotherapy) | 2 sessions/day | 8 weeks | Continuous respiratory muscle exercise or continuous aerobic exercise | CPET (VO2peak, maximum working load pred, maximum ventilation, maximum inspiratory pressure, and maximum expiratory pressure) | (88) |
Tlco, carbon monoxide transfer factor; CPET, cardiopulmonary exercise testing; CAT, COPD Assessment Test; chronic obstructive pulmonary disease; DLCO, diffusing capacity of the lungs for carbon monoxide; MVV, maximal voluntary ventilation; SF-36, 36-item Short-Form; EORTC-QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30; 6MWT, 6-minute walk test; FACT-L, Functional Assessment of Lung Cancer Therapy-Lung; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; mMRC, modified British Medical Research Council Dyspnea Scale; PAL, physical activity level; RPE, rating of perceived exertion.
According to the CSCO guidelines, NCCN guidelines, and findings from currently available clinical research, postoperative adjuvant therapies typically include chemotherapy, radiotherapy, and targeted therapy. For patients without epidermal growth factor receptor (EGFR)-sensitive mutations, postoperative adjuvant chemotherapy is preferred. For patients with EGFR-sensitive mutations, postoperative adjuvant targeted therapy with osimertinib can also be selected. Many clinical trials on postoperative adjuvant targeted therapies and postoperative adjuvant immunotherapy are still on the way.
For patients with completely resected small cell lung cancer (SCLC) (N0 or N1) combined with COPD, postoperative adjuvant chemotherapy is required (evidence level: 2A). The current evidence for surgical treatment and perioperative management of SCLC comes from the US National Cancer Database (NCDB). Since patients with mild to moderate COPD are not ruled out in the database, postoperative adjuvant therapy of patients with SCLC combined with COPD can also refer to the NCCN guidelines and the CSCO guidelines (90-92). A platinum-based dual-drug regimen is recommended for postoperative adjuvant chemotherapy, with etoposide being a fundamental and essential part of the combinations. It is recommended that postoperative adjuvant chemotherapy be started when the physical status has basically returned to normal (PS score is less than 2, COPD is in a stable stage, and respiratory function has recovered), usually 4 to 6 weeks after surgery but typically no later than 3 months after surgery. Four cycles of postoperative adjuvant chemotherapy are routinely recommended. In addition, the large amount of rehydration after cisplatin administration requires adequate cardiac function. For patients with moderate COPD, carboplatin-based dual-drug regimens are recommended for postoperative adjuvant chemotherapy. Since leukopenia is a common toxicity of chemotherapy, COPD patients have an increased risk of acute exacerbation of COPD due to infection after chemotherapy. Therefore, efforts should be made to avoid infection during PR.
For patients with completely resected SCLC (N2 or N1) combined with COPD and those with uncompleted resected SCLC combined with COPD, postoperative adjuvant chemotherapy plus radiotherapy is required (evidence level: 2A). The current evidence for surgical treatment and perioperative management of SCLC comes from the US NCDB. Since patients with mild to moderate COPD are not ruled out in the database, postoperative adjuvant therapy of patients with SCLC combined with COPD can also refer to the NCCN guidelines and the CSCO guidelines (90-92). A platinum-based dual-drug regimen is recommended for postoperative adjuvant chemotherapy, with etoposide being a fundamental and essential part of the combinations. At present, the recommended adjuvant radiotherapy strategies include three-dimensional conformal radiotherapy, intensity-modulated radiotherapy, and volumetric modulated arc therapy, with the target areas mainly including the ipsilateral hilum, ipsilateral mediastinum, subcarina, and other high-risk areas for local recurrence. The CSCO guidelines recommend a total dose of 50 Gy. There are no independent data on the safety and toxicities of adjuvant chemotherapy combined with adjuvant radiotherapy after SCLC surgery. Referring to the results of adjuvant chemotherapy combined with adjuvant radiotherapy after an NSCLC surgery, we assume that the incidence of severe radiation pneumonitis after postoperative adjuvant radiotherapy is about 1%. Radiation pneumonitis is a common adverse reaction of radiotherapy. For mild patients who do not need glucocorticoid therapy, radiation pneumonitis does not affect the implementation of PR. However, for severe radiation pneumonitis patients who require glucocorticoid therapy, PR should be suspended immediately, along with glucocorticoid, oxygen, antibiotics, and other symptomatic treatments. After the radiation pneumonia is improved, PR may be resumed after evaluation by health professionals.
For completely resected stage IA NSCLC patients with COPD and for completely resected stage IB NSCLC (without high risks) patients with COPD, no adjuvant therapy is required after surgical treatment, and the mainstay of management is regular follow-up. Treatment can be performed according to the established PR strategies, followed by reassessment.
Postoperative adjuvant chemotherapy is mainly indicated for stage IB NSCLC patients with high-risk factors and for stage II–III NSCLC patients. Notably, patients with mild to moderate COPD are not excluded in the current clinical trials on postoperative adjuvant radiotherapy (93-96). Therefore, postoperative adjuvant chemotherapy for NSCLC patients with mild and moderate COPD may refer to the CSCO guidelines and NCCN guidelines. A platinum-based dual-drug regimen is recommended for postoperative adjuvant chemotherapy, with vinorelbine, gemcitabine, docetaxel, paclitaxel, nab-paclitaxel, and pemetrexed (for non-squamous lesions) being a fundamental and essential part of the combinations. It is recommended that postoperative adjuvant chemotherapy be started when the physical status has basically returned to normal (PS score is less than 2, COPD is in a stable stage, and respiratory function has recovered), usually 4 to 6 weeks after surgery but typically no later than 3 months after surgery. Four cycles of postoperative adjuvant chemotherapy are routinely recommended. In addition, the large amount of rehydration after cisplatin administration requires adequate cardiac function. For patients with moderate COPD, carboplatin-based dual-drug regimens are recommended for postoperative adjuvant chemotherapy. Since leukopenia is a common toxicity of chemotherapy, COPD patients have an increased risk of acute exacerbation of COPD due to infection after chemotherapy. For stage IB (with high-risk factors) NSCLC patients with moderate COPD, experts recommend that postoperative adjuvant chemotherapy should be waived or only reduced-dose chemotherapy is applied.
The timing of PR during postoperative adjuvant chemotherapy for patients undergoing postoperative adjuvant chemotherapy remains controversial. In Cavalheri’s study, PR was carried out after adjuvant chemotherapy was completed, while in Hoffman’s study, PR was initiated 1 month after surgery, regardless of whether chemotherapy was performed (87,97). An 8-week PR program at the outpatient department may be feasible for patients who have received chemotherapy for advanced lung cancer, according to a cohort study (88). Ozalevli suggested that PR in patients with advanced lung cancer during chemotherapy can improve exercise capacity and quality of life, alleviate dyspnea, relieve anxiety (98). When designing PR programs (especially exercise), attention should be paid to the toxicities of chemotherapy. For example, patients receiving paclitaxel treatment may be at risk of arrhythmia or blood pressure changes, in whom cardiovascular function should be closely monitored during exercise, and a medical referral will be required if discomfort occurs. Chemotherapy often results in bone marrow suppression; therefore, trauma should be avoided during exercise for patients experiencing thrombocytopenia and infection should be prevented in patients suffering from leukopenia.
Recent studies such as ADJUVANT and EVAN have shown that adjuvant targeted therapy further improves the disease-free survival (DFS), although definite data on the overall survival (OS) are not yet available (99-101). In April 2021, following its global approval, osimertinib was approved in China as an adjuvant therapy for stage IB–IIIA NSCLC with EGFR mutations, becoming the first targeted drug approved for the adjuvant treatment of lung cancer in China and abroad (102). The main adverse reactions of postoperative adjuvant targeted therapy in clinical trials include rash, abnormal liver function, dry skin, and diarrhea. Notably, the existing large-scale clinical studies have not excluded COPD patients. Therefore, for patients with early-stage NSCLC accompanied by mild to moderate COPD, the choice of postoperative adjuvant targeted therapy can refer to the CSCO and NCCN guidelines, which have suggested that adjuvant targeted therapy is more feasible for high-risk patients with stage IB NSCLC and for postoperative stage II–III NSCLC patients with EGFR-sensitive mutations.
No literature has described the value of PR in this population. The side effects of targeted therapy are typically mild and will not affect lung function or quality of life. There is generally no need to adjust PR strategies for these patients. We recommend that these patients may carry out PR exercises according to the routine PR mode. If pulmonary fibrosis occurs, in addition to measures such as drug withdrawal, exercise training may be conducted with reference to the rehabilitation strategies for interstitial lung disease.
The clinical pathway of postoperative adjuvant radiotherapy can refer to the CSCO and NCCN guidelines, mainly applicable to patients undergoing non-radical surgery and those with N2 tumors (95,103,104). Notably, patients with mild to moderate COPD are not excluded in the current clinical trials on postoperative adjuvant radiotherapy. Clinical research has indicated that the incidence of severe radiation pneumonitis following postoperative adjuvant radiotherapy is about 1%. Although this complication is uncommon, it can be life-threatening if not managed properly. For patients with moderate COPD, the incidence of radiation pneumonitis after postoperative adjuvant radiotherapy is still unclear, and the clinical benefit-risk profile needs to be further investigated. They must be fully informed of possible adverse reactions when choosing postoperative adjuvant radiotherapy. Meanwhile, PR may be performed before radiation therapy as it can alleviate the side effects of radiation therapy. For patients receiving stereotactic body radiation therapy (SBRT), since the treatment has no significant effect on lung function, it can be carried out concurrently with PR.
Adverse reactions such as skin injuries and leukopenia may occur during postoperative adjuvant radiotherapy. Therefore, wound infection should be avoided during PR. Radiation pneumonitis is a common adverse reaction of radiotherapy. For mild patients who do not need glucocorticoid therapy, radiation pneumonitis does not affect the implementation of PR. However, for severe radiation pneumonitis patients who require glucocorticoid therapy, PR should be suspended immediately, along with the use of glucocorticoid, oxygen, antibiotics, and other symptomatic treatments. After the radiation pneumonia is improved, PR may be resumed after evaluation by healthcare professionals.
Acknowledgments
Funding: This study was supported by the National Key Research and Development Program of China (No. 2018YFC1313600), National Natural Science Foundation of China (No. 82150005) and Chinese Society of Clinical Oncology (CSCO) (No. Y-2019AZZD-0038).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-549/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zheng R, Zhang S, Zeng H, et al. Cancer incidence and mortality in China, 2016. J Natl Cancer Cent 2022;2:1-9. [Crossref]
- Chinese Thoracic Society, Chinese Association of Chest Physicians. Guidelines for the diagnosis and management of chronic obstructive pulmonary disease (revised version 2021). Chinese Journal of Tuberculosis and Respiratory Diseases 2021;44:170-205. [PubMed]
- Zeneyedpour L, Dekker LJM, van Sten-van T, Hoff JJM, et al. Neoantigens in Chronic Obstructive Pulmonary Disease and Lung Cancer: A Point of View. Proteomics Clin Appl 2019;13:e1800093. [Crossref] [PubMed]
- Hamilton G, Rath B. Smoking, inflammation and small cell lung cancer: recent developments. Wien Med Wochenschr 2015;165:379-86. [Crossref] [PubMed]
- Singh N, Baby D, Rajguru JP, et al. Inflammation and cancer. Ann Afr Med 2019;18:121-6. [Crossref] [PubMed]
- Houghton AM. Common Mechanisms Linking Chronic Obstructive Pulmonary Disease and Lung Cancer. Ann Am Thorac Soc 2018;15:S273-7. [Crossref] [PubMed]
- Young RP, Hopkins RJ, Christmas T, et al. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J 2009;34:380-6. [Crossref] [PubMed]
- de Torres JP, Marín JM, Casanova C, et al. Lung cancer in patients with chronic obstructive pulmonary disease-- incidence and predicting factors. Am J Respir Crit Care Med 2011;184:913-9. [Crossref] [PubMed]
- David EA, Clark JM, Cooke DT, et al. The Role of Thoracic Surgery in the Therapeutic Management of Metastatic Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:1636-45. [Crossref] [PubMed]
- Miyazawa M, Haniuda M, Nishimura H, et al. Longterm effects of pulmonary resection on cardiopulmonary function. J Am Coll Surg 1999;189:26-33. [Crossref] [PubMed]
- Schulte T, Schniewind B, Dohrmann P, et al. The extent of lung parenchyma resection significantly impacts long-term quality of life in patients with non-small cell lung cancer. Chest 2009;135:322-9. [Crossref] [PubMed]
- Gullón JA, Suárez I, Medina A, et al. Lung cancer: changes in epidemiology and survival. Rev Clin Esp 2012;212:18-23. [PubMed]
- Jones LW. Physical activity and lung cancer survivorship. Recent Results Cancer Res 2011;186:255-74. [Crossref] [PubMed]
- Mao X, Zhang W, Ni YQ, et al. A Prediction Model for Postoperative Pulmonary Complication in Pulmonary Function-Impaired Patients Following Lung Resection. J Multidiscip Healthc 2021;14:3187-94. [Crossref] [PubMed]
- Rochester CL, Vogiatzis I, Holland AE, et al. An Official American Thoracic Society/European Respiratory Society Policy Statement: Enhancing Implementation, Use, and Delivery of Pulmonary Rehabilitation. Am J Respir Crit Care Med 2015;192:1373-86. [Crossref] [PubMed]
- Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013;188:e13-64. [Crossref] [PubMed]
- Markos J, Mullan BP, Hillman DR, et al. Preoperative assessment as a predictor of mortality and morbidity after lung resection. Am Rev Respir Dis 1989;139:902-10. [Crossref] [PubMed]
- Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011;43:1334-59. [Crossref] [PubMed]
- Horowitz MB, Littenberg B, Mahler DA. Dyspnea ratings for prescribing exercise intensity in patients with COPD. Chest 1996;109:1169-75. [Crossref] [PubMed]
- Lacasse Y, Goldstein R, Lasserson TJ, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006;CD003793. [PubMed]
- O'Donnell DE, McGuire M, Samis L, et al. General exercise training improves ventilatory and peripheral muscle strength and endurance in chronic airflow limitation. Am J Respir Crit Care Med 1998;157:1489-97. [Crossref] [PubMed]
- Casaburi R, Porszasz J, Burns MR, et al. Physiologic benefits of exercise training in rehabilitation of patients with severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1997;155:1541-51. [Crossref] [PubMed]
- Maltais F, LeBlanc P, Jobin J, et al. Intensity of training and physiologic adaptation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1997;155:555-61. [Crossref] [PubMed]
- Jenkins S, Hill K, Cecins NM. State of the art: how to set up a pulmonary rehabilitation program. Respirology 2010;15:1157-73. [Crossref] [PubMed]
- Vogiatzis I, Nanas S, Roussos C. Interval training as an alternative modality to continuous exercise in patients with COPD. Eur Respir J 2002;20:12-9. [Crossref] [PubMed]
- Vogiatzis I, Terzis G, Nanas S, et al. Skeletal muscle adaptations to interval training in patients with advanced COPD. Chest 2005;128:3838-45. [Crossref] [PubMed]
- American College of Sports Medicine. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc 2009;41:687-708. [Crossref] [PubMed]
- Williams MA, Haskell WL, Ades PA, et al. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation 2007;116:572-84. [Crossref] [PubMed]
- Probst VS, Troosters T, Pitta F, et al. Cardiopulmonary stress during exercise training in patients with COPD. Eur Respir J 2006;27:1110-8. [Crossref] [PubMed]
- Annegarn J, Meijer K, Passos VL, et al. Problematic activities of daily life are weakly associated with clinical characteristics in COPD. J Am Med Dir Assoc 2012;13:284-90. [Crossref] [PubMed]
- Janaudis-Ferreira T, Hill K, Goldstein R, et al. Arm exercise training in patients with chronic obstructive pulmonary disease: a systematic review. J Cardiopulm Rehabil Prev 2009;29:277-83. [Crossref] [PubMed]
- Sandsund CA, Roughton M, Hodson ME, et al. Musculoskeletal techniques for clinically stable adults with cystic fibrosis: a preliminary randomised controlled trial. Physiotherapy 2011;97:209-17. [Crossref] [PubMed]
- Eberstein A, Eberstein S. Electrical stimulation of denervated muscle: is it worthwhile? Med Sci Sports Exerc 1996;28:1463-9. [Crossref] [PubMed]
- Mathur S, Hornblower E, Levy RD. Exercise training before and after lung transplantation. Phys Sportsmed 2009;37:78-87. [Crossref] [PubMed]
- Beaumont M, Forget P, Couturaud F, et al. Effects of inspiratory muscle training in COPD patients: A systematic review and meta-analysis. Clin Respir J 2018;12:2178-88. [Crossref] [PubMed]
- Geddes EL, O'Brien K, Reid WD, et al. Inspiratory muscle training in adults with chronic obstructive pulmonary disease: an update of a systematic review. Respir Med 2008;102:1715-29. [Crossref] [PubMed]
- Gosselink R, De Vos J, van den Heuvel SP, et al. Impact of inspiratory muscle training in patients with COPD: what is the evidence? Eur Respir J 2011;37:416-25. [Crossref] [PubMed]
- Sillen MJH, Speksnijder CM, Eterman RA, et al. Effects of neuromuscular electrical stimulation of muscles of ambulation in patients with chronic heart failure or COPD: a systematic review of the English-language literature. Chest 2009;136:44-61. [Crossref] [PubMed]
- Quittan M, Wiesinger GF, Sturm B, et al. Improvement of thigh muscles by neuromuscular electrical stimulation in patients with refractory heart failure: a single-blind, randomized, controlled trial. Am J Phys Med Rehabil 2001;80:206-14; quiz 215-6, 224.
- Sillen MJ, Janssen PP, Akkermans MA, et al. The metabolic response during resistance training and neuromuscular electrical stimulation (NMES) in patients with COPD, a pilot study. Respir Med 2008;102:786-9. [Crossref] [PubMed]
- Venkatesan P. GOLD report: 2022 update. Lancet Respir Med 2022;10:e20. [Crossref] [PubMed]
- O'Donnell DE, Flüge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J 2004;23:832-40. [Crossref] [PubMed]
- Liesker JJ, Wijkstra PJ, Ten Hacken NH, et al. A systematic review of the effects of bronchodilators on exercise capacity in patients with COPD. Chest 2002;121:597-608. [Crossref] [PubMed]
- Ferreira IM, Verreschi IT, Nery LE, et al. The influence of 6 months of oral anabolic steroids on body mass and respiratory muscles in undernourished COPD patients. Chest 1998;114:19-28. [Crossref] [PubMed]
- Schols AM, Soeters PB, Mostert R, et al. Physiologic effects of nutritional support and anabolic steroids in patients with chronic obstructive pulmonary disease. A placebo-controlled randomized trial. Am J Respir Crit Care Med 1995;152:1268-74. [Crossref] [PubMed]
- Yeh SS, DeGuzman B, Kramer T, et al. Reversal of COPD-associated weight loss using the anabolic agent oxandrolone. Chest 2002;122:421-8. [Crossref] [PubMed]
- Burdet L, de Muralt B, Schutz Y, et al. Administration of growth hormone to underweight patients with chronic obstructive pulmonary disease. A prospective, randomized, controlled study. Am J Respir Crit Care Med 1997;156:1800-6. [Crossref] [PubMed]
- Pape GS, Friedman M, Underwood LE, et al. The effect of growth hormone on weight gain and pulmonary function in patients with chronic obstructive lung disease. Chest 1991;99:1495-500. [Crossref] [PubMed]
- Garrod R, Paul EA, Wedzicha JA. Supplemental oxygen during pulmonary rehabilitation in patients with COPD with exercise hypoxaemia. Thorax 2000;55:539-43. [Crossref] [PubMed]
- Weisberg J, Wanger J, Olson J, et al. Megestrol acetate stimulates weight gain and ventilation in underweight COPD patients. Chest 2002;121:1070-8. [Crossref] [PubMed]
- Nagaya N, Itoh T, Murakami S, et al. Treatment of cachexia with ghrelin in patients with COPD. Chest 2005;128:1187-93. [Crossref] [PubMed]
- Héraud N, Préfaut C, Durand F, et al. Does correction of exercise-induced desaturation by O(2) always improve exercise tolerance in COPD? A preliminary study. Respir Med 2008;102:1276-86. [Crossref] [PubMed]
- Schols AM, Mostert R, Soeters PB, et al. Body composition and exercise performance in patients with chronic obstructive pulmonary disease. Thorax 1991;46:695-9. [Crossref] [PubMed]
- Abrahm JL, Hansen-Flaschen J. Hospice care for patients with advanced lung disease. Chest 2002;121:220-9. [Crossref] [PubMed]
- Pison CM, Cano NJ, Chérion C, et al. Multimodal nutritional rehabilitation improves clinical outcomes of malnourished patients with chronic respiratory failure: a randomised controlled trial. Thorax 2011;66:953-60. [Crossref] [PubMed]
- van Wetering CR, Hoogendoorn M, Broekhuizen R, et al. Efficacy and costs of nutritional rehabilitation in muscle-wasted patients with chronic obstructive pulmonary disease in a community-based setting: a prespecified subgroup analysis of the INTERCOM trial. J Am Med Dir Assoc 2010;11:179-87. [Crossref] [PubMed]
- Weekes CE, Emery PW, Elia M. Dietary counselling and food fortification in stable COPD: a randomised trial. Thorax 2009;64:326-31. [Crossref] [PubMed]
- Agostini P, Cieslik H, Rathinam S, et al. Postoperative pulmonary complications following thoracic surgery: are there any modifiable risk factors? Thorax 2010;65:815-8. [Crossref] [PubMed]
- Maeda R, Yoshida J, Ishii G, et al. The prognostic impact of cigarette smoking on patients with non-small cell lung cancer. J Thorac Oncol 2011;6:735-42. [Crossref] [PubMed]
- Lugg ST, Tikka T, Agostini PJ, et al. Smoking and timing of cessation on postoperative pulmonary complications after curative-intent lung cancer surgery. J Cardiothorac Surg 2017;12:52. [Crossref] [PubMed]
- Sheikh M, Mukeriya A, Shangina O, et al. Postdiagnosis Smoking Cessation and Reduced Risk for Lung Cancer Progression and Mortality: A Prospective Cohort Study. Ann Intern Med 2021;174:1232-9. [Crossref] [PubMed]
- Fritzsche A, Clamor A, von Leupoldt A. Effects of medical and psychological treatment of depression in patients with COPD--a review. Respir Med 2011;105:1422-33. [Crossref] [PubMed]
- Neuringer A. Operant variability: evidence, functions, and theory. Psychon Bull Rev 2002;9:672-705. [Crossref] [PubMed]
- Bandura A. Social learning of moral judgments. J Pers Soc Psychol 1969;11:275-9. [Crossref] [PubMed]
- Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev 1977;84:191-215. [Crossref] [PubMed]
- Adams SG, Smith PK, Allan PF, et al. Systematic review of the chronic care model in chronic obstructive pulmonary disease prevention and management. Arch Intern Med 2007;167:551-61. [Crossref] [PubMed]
- Bobbio A, Chetta A, Ampollini L, et al. Preoperative pulmonary rehabilitation in patients undergoing lung resection for non-small cell lung cancer. Eur J Cardiothorac Surg 2008;33:95-8. [Crossref] [PubMed]
- Divisi D, Di Francesco C, Di Leonardo G, et al. Preoperative pulmonary rehabilitation in patients with lung cancer and chronic obstructive pulmonary disease. Eur J Cardiothorac Surg 2013;43:293-6. [Crossref] [PubMed]
- Coats V, Maltais F, Simard S, et al. Feasibility and effectiveness of a home-based exercise training program before lung resection surgery. Can Respir J 2013;20:e10-6. [Crossref] [PubMed]
- Stefanelli F, Meoli I, Cobuccio R, et al. High-intensity training and cardiopulmonary exercise testing in patients with chronic obstructive pulmonary disease and non-small-cell lung cancer undergoing lobectomy. Eur J Cardiothorac Surg 2013;44:e260-5. [Crossref] [PubMed]
- Morano MT, Araújo AS, Nascimento FB, et al. Preoperative pulmonary rehabilitation versus chest physical therapy in patients undergoing lung cancer resection: a pilot randomized controlled trial. Arch Phys Med Rehabil 2013;94:53-8. [Crossref] [PubMed]
- Mujovic N, Mujovic N, Subotic D, et al. Preoperative pulmonary rehabilitation in patients with non-small cell lung cancer and chronic obstructive pulmonary disease. Arch Med Sci 2014;10:68-75. [Crossref] [PubMed]
- Morano MT, Mesquita R, Da Silva GP, et al. Comparison of the effects of pulmonary rehabilitation with chest physical therapy on the levels of fibrinogen and albumin in patients with lung cancer awaiting lung resection: a randomized clinical trial. BMC Pulm Med 2014;14:121. [Crossref] [PubMed]
- Tarumi S, Yokomise H, Gotoh M, et al. Pulmonary rehabilitation during induction chemoradiotherapy for lung cancer improves pulmonary function. J Thorac Cardiovasc Surg 2015;149:569-73. [Crossref] [PubMed]
- Mujovic N, Mujovic N, Subotic D, et al. Influence of Pulmonary Rehabilitation on Lung Function Changes After the Lung Resection for Primary Lung Cancer in Patients with Chronic Obstructive Pulmonary Disease. Aging Dis 2015;6:466-77. [Crossref] [PubMed]
- Vagvolgyi A, Rozgonyi Z, Kerti M, et al. Effectiveness of perioperative pulmonary rehabilitation in thoracic surgery. J Thorac Dis 2017;9:1584-91. [Crossref] [PubMed]
- Hashmi A, Baciewicz FA Jr, Soubani AO, et al. Preoperative pulmonary rehabilitation for marginal-function lung cancer patients. Asian Cardiovasc Thorac Ann 2017;25:47-51. [Crossref] [PubMed]
- Lai Y, Su J, Qiu P, et al. Systematic short-term pulmonary rehabilitation before lung cancer lobectomy: a randomized trial. Interact Cardiovasc Thorac Surg 2017;25:476-83. [Crossref] [PubMed]
- Lai Y, Huang J, Yang M, et al. Seven-day intensive preoperative rehabilitation for elderly patients with lung cancer: a randomized controlled trial. J Surg Res 2017;209:30-6. [Crossref] [PubMed]
- Vagvolgyi A, Rozgonyi Z, Kerti M, et al. Effectiveness of pulmonary rehabilitation and correlations in between functional parameters, extent of thoracic surgery and severity of post-operative complications: randomized clinical trial. J Thorac Dis 2018;10:3519-31. [Crossref] [PubMed]
- Marhic A, Dakhil B, Plantefeve G, et al. Long-term survival following lung surgery for cancer in high-risk patients after perioperative pulmonary rehabilitation. Interact Cardiovasc Thorac Surg 2019;28:235-9. [Crossref] [PubMed]
- Kwong KF, Edelman MJ, Suntharalingam M, et al. High-dose radiotherapy in trimodality treatment of Pancoast tumors results in high pathologic complete response rates and excellent long-term survival. J Thorac Cardiovasc Surg 2005;129:1250-7. [Crossref] [PubMed]
- Rusch VW, Giroux DJ, Kraut MJ, et al. Induction chemoradiation and surgical resection for superior sulcus non-small-cell lung carcinomas: long-term results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160). J Clin Oncol 2007;25:313-8. [Crossref] [PubMed]
- Rusch VW, Giroux DJ, Kraut MJ, et al. Induction chemoradiation and surgical resection for non-small cell lung carcinomas of the superior sulcus: Initial results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160). J Thorac Cardiovasc Surg 2001;121:472-83. [Crossref] [PubMed]
- Edvardsen E, Skjønsberg OH, Holme I, et al. High-intensity training following lung cancer surgery: a randomised controlled trial. Thorax 2015;70:244-50. [Crossref] [PubMed]
- Maeda K, Higashimoto Y, Honda N, et al. Effect of a postoperative outpatient pulmonary rehabilitation program on physical activity in patients who underwent pulmonary resection for lung cancer. Geriatr Gerontol Int 2016;16:550-5. [Crossref] [PubMed]
- Cavalheri V, Jenkins S, Cecins N, et al. Exercise training for people following curative intent treatment for non-small cell lung cancer: a randomized controlled trial. Braz J Phys Ther 2017;21:58-68. [Crossref] [PubMed]
- Messaggi-Sartor M, Marco E, Martínez-Téllez E, et al. Combined aerobic exercise and high-intensity respiratory muscle training in patients surgically treated for non-small cell lung cancer: a pilot randomized clinical trial. Eur J Phys Rehabil Med 2019;55:113-22. [Crossref] [PubMed]
- Quist M, Sommer MS, Vibe-Petersen J, et al. Early initiated postoperative rehabilitation reduces fatigue in patients with operable lung cancer: A randomized trial. Lung Cancer 2018;126:125-32. [Crossref] [PubMed]
- Yang CJ, Chan DY, Shah SA, et al. Long-term Survival After Surgery Compared With Concurrent Chemoradiation for Node-negative Small Cell Lung Cancer. Ann Surg 2018;268:1105-12. [Crossref] [PubMed]
- Yang CF, Chan DY, Speicher PJ, et al. Role of Adjuvant Therapy in a Population-Based Cohort of Patients With Early-Stage Small-Cell Lung Cancer. J Clin Oncol 2016;34:1057-64. [Crossref] [PubMed]
- Yang CJ, Chan DY, Speicher PJ, et al. Surgery Versus Optimal Medical Management for N1 Small Cell Lung Cancer. Ann Thorac Surg 2017;103:1767-72. [Crossref] [PubMed]
- Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 2004;350:351-60. [Crossref] [PubMed]
- Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med 2005;352:2589-97. [Crossref] [PubMed]
- Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association ANITA): a randomised controlled trial. Lancet Oncol 2006;7:719-27. [Crossref] [PubMed]
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [Crossref] [PubMed]
- Hoffman AJ, Brintnall RA, von Eye A, et al. Home-based exercise: promising rehabilitation for symptom relief, improved functional status and quality of life for post-surgical lung cancer patients. J Thorac Dis 2014;6:632-40. [PubMed]
- Ozalevli S. Impact of physiotherapy on patients with advanced lung cancer. Chron Respir Dis 2013;10:223-32. [Crossref] [PubMed]
- Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol 2018;19:139-48. [Crossref] [PubMed]
- Yue D, Xu S, Wang Q, et al. Erlotinib versus vinorelbine plus cisplatin as adjuvant therapy in Chinese patients with stage IIIA EGFR mutation-positive non-small-cell lung cancer (EVAN): a randomised, open-label, phase 2 trial. Lancet Respir Med 2018;6:863-73. [Crossref] [PubMed]
- He J, Su C, Liang W, et al. Icotinib versus chemotherapy as adjuvant treatment for stage II-IIIA EGFR-mutant non-small-cell lung cancer (EVIDENCE): a randomised, open-label, phase 3 trial. Lancet Respir Med 2021;9:1021-9. [Crossref] [PubMed]
- Wu YL, Tsuboi M, He J, et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N Engl J Med 2020;383:1711-23. [Crossref] [PubMed]
- Dillman RO, Seagren SL, Propert KJ, et al. A randomized trial of induction chemotherapy plus high-dose radiation versus radiation alone in stage III non-small-cell lung cancer. N Engl J Med 1990;323:940-5. [Crossref] [PubMed]
- Lally BE, Zelterman D, Colasanto JM, et al. Postoperative radiotherapy for stage II or III non-small-cell lung cancer using the surveillance, epidemiology, and end results database. J Clin Oncol 2006;24:2998-3006. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)


