Editor’s note: “Palliative Radiotherapy Column” features articles emphasizing the critical role of radiotherapy in palliative care. Chairs to the columns are Dr. Edward L. W. Chow from Odette Cancer Centre, Sunnybrook Health Sciences Centre in Toronto and Dr. Stephen Lutz from Blanchard Valley Regional Cancer Center in Findlay, gathering a group of promising researchers in the field to make it an excellent column. The column includes original research manuscripts and timely review articles and perspectives relating to palliative radiotherapy, as well as editorials and commentaries on recently published trials and studies.
Urinary cytokines/chemokines pattern in patients with painful bone metastases undergoing external beam radiotherapy experiencing pain flare
Introduction
Bone metastases are a common occurrence in patients with metastatic cancer. Cancer induced bone pain (CIBP) is a complex phenomenon mediated by the combined effects of locally produced cytokines, local primary afferent nerve fibers and the central nervous system (1). Pain is experienced by 50% to 75% of patients with bone metastases (1). Recent evidence-based guidelines reaffirm external beam radiotherapy (EBRT) as the mainstay for treatment of painful bone metastases (2). The overall pain response rate is approximately 60% (3). Worsening of pain (“pain flare”) within 10 days of radiotherapy to bone metastases occurs in up to 40% of patients (4). The pathophysiology of pain flare is poorly understood (5). We postulated that inflammatory mediators play a role in pain flare.
The inflammatory markers are often measured in serum, but can also be measured in urine samples, which is preferable for our palliative patient population. This has been demonstrated in several studies using enzyme-linked immunosorbent assay (ELISA) for analysis of these cytokines (6,7). A recent study by Sirera et al demonstrated no statistically significant difference in levels of IL-6 and TNFα measured in serum and urine (6). We investigated the pathophysiology of pain flare through assessment of changes in urinary cytokines/chemokines in patients receiving EBRT for painful bone metastases to better understand the mechanism of pain flare.
Methods
Eligible cancer patients with bone metastases treated with single fraction EBRT at Odettte Cancer Centre (OCC) were approached. Inclusion criteria included pathological diagnosis of cancer, radiological evidence of bone metastases (e.g., bone scan, plain X-ray, CT or MRI scans) and treatment with a single 8 Gy fraction. Patients were able to understand English and complete the interview/diary, as well as willing to complete the daily diary on pain score and analgesic intake before, daily during radiation, and 10 days following the completion of radiation treatment. Patients were to give a written consent to enrollment. Exclusion criteria included pathological fracture at the irradiated site, spinal cord or cauda equina compression, patients taking any form of steroids within a week of the start of radiation, planned steroid during the radiation and then 10 days following the completion of radiotherapy, planned surgery, start or change of bisphosphonates and/or systemic therapy during the radiation and the 10 days following the completion of radiotherapy.
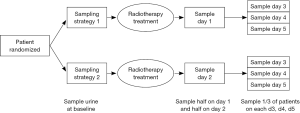
Ethics approval was obtained from the Research Ethics Board at Sunnybrook Health Sciences Centre. Urine samples were collected from patients receiving a single 8 Gy radiotherapy for painful bone metastases at baseline (day of EBRT), day 1 or 2 and on an additional day between days 3 to 5 post radiation (Figure 1).
Patients completed a standardized pain and analgesic use diary daily for 10 days following the day of radiation. Completing the diary could be done independently or alternatively through the research assistant who could contact the patient daily by telephone to collect the data.
Patients were deemed to have pain flare if they had a two-point increase from baseline pain on 0–10 scale and no decrease in analgesic intake or a 25% increase in analgesic intake measured by daily oral morphine equivalent (OME) with no decrease in pain (8). In addition the pain score and analgesic intake had to return back to baseline levels after the flare. Pain flare was distinguished from progression of pain by returning of the worst pain score and analgesic intake to baseline level within the 10 days follow up period.
Given the underlying medical condition for palliative patients, we decided to use the least invasive method to measure cytokines/chemokines. The Millipore Milliplex 42-Plex Cyto-kine/Chemokine Kit™ was used to measure urinary levels of a panel of cytokines/chemokines. Cytokine levels were corrected according to urine creatinine level. In each urine sample we measured EGF, eotaxin, FGF-2, Flt-3 ligand, fractalkine, G-CSF, GM-CSF, GRO, INFα2, INFγ, IL-1ra, IL-1α, IL-1β, IL-2, sIL-2Rα, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12(p40), IL-12(p70), IL-13, IL-15, IL-17A, interferon gamma induced protein 10 (IP-10), MCP-1, MCP-3, macrophage derived chemokine (MDC), MIP-1α, MIP-1β, PDGF-AA, PDGF-AB/BB, RANTES, sCD40L, TGFα, TNFα, TNFβ and VEGF as well as markers of bone turnover (N-telopeptides).
Patients were randomized in blocks of three to one of the following groups of urine sample collection time:
- Group A—sample on day 1 and day 3;
- Group B—sample on day 2 and day 3;
- Group C—sample on day 1 and day 4;
- Group D—sample on day 2 and day 4;
- Group E—sample on day 1 and day 5;
- Group F—sample on day 2 and day 5.
Statistical analysis
Using a random number generator, we randomly allocated patients in a two-step process first to either sampling strategy 1 or 2 and then to 1 of 3 urine collection strategies for the second urine sample to limit the number of urine samples the patients had to provide and at the same time having urine samples from the group of patients covering baseline through to 5 days after treatment. At the time of study planning there was no data to indicate if and when a cytokine may increase in amount in the urine so we wanted to cover up to 5 days post radiation for the population.
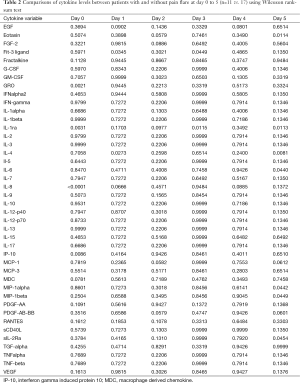
Demographics were summarized in all patients and in patients with pain flare, using mean, standard deviation (SD), median, interquartiles (Q1, Q3), and range for continuous variables; and proportions for categorical variables. Wilcoxon Rank-sum nonparametric test was applied for comparing each cytokine levels between pain flare and non-pain flare at baseline and day 1–5, respectively. To search for significant time trends for each cytokine variable from baseline to day 5 and investigate if pain flare patients had different time trends compared to non-pain flare patients, general linear regression analysis was conducted on each cytokine variable. To normalize the cytokine distribution, natural log-transformation was applied for all cytokine variables. The outcomes were corrected cytokine levels (log scale), the independent variables including time (from baseline to day 5), binary variable of pain flare (1= yes, 0= no), and the interaction term between post treatment day and pain flare. Heat maps were created for 42 cytokine variables in all patients and in patients with or without pain flare. Box plots of percent change from baseline were generated for EGF, IL-8, IP-10, and MDC in all patients and in patients with or without pain flare. All analyses were conducted using Statistical Analysis Software (SAS version 9.4 for Windows), and P value <0.05 was considered statistically significant.
Results
From January 2010 to May 2012, 46 patients consented for the study. Of those, we had 28 patients with complete data. The most common reason for exclusion was incomplete data in the pain diary. Other reasons included the deteriorating health or death of the patients for which samples were not included in the study. There were no statistically significant differences between enrolled and excluded patients in terms of gender, age, primary cancer site, baseline pain score, baseline analgesic consumtion or previous systemic therapy. None of the patients recruited took non steroidal anti-inflammatory agents during the study period.
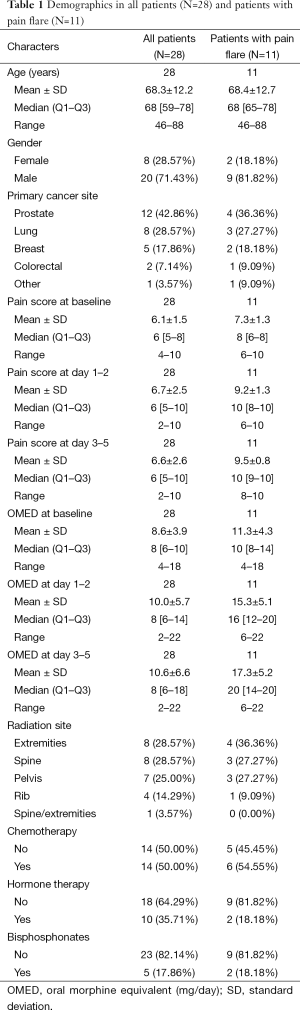
Table 1 summarises the demographics for 28 patients enrolled. The median age of enrolled patients was 68 years. There were 20 males (71%) and 8 females (29%). The most common primary cancer site was prostate (43%) followed by lung (29%) and breast (19%). Out of the 28 patients enrolled, 11 (39%) developed pain flare (Table 1). Nine were males (82%) and 2 were females (18%). For this group, prostate was the most common primary cancer site (36%), followed by lung (27%) and breast (18%).
Full table
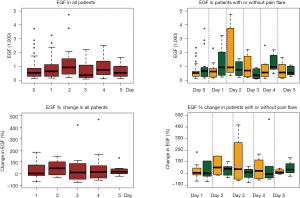
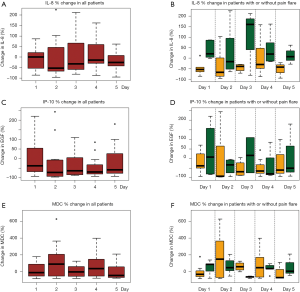
All available urine samples for analyzing 42 cytokine variables at baseline, and days 1–5 were used. Each cytokine variable was compared between patients with pain flare and patients without pain flare at baseline and day 1–5, respectively (Table 2). Figure 2 shows that the levels of cytokines/chemokines detectable in the urine varied significantly in all patients and in patients with or without pain flare, respectively. The following cytokines/chemokines were detectable in at least 50% of the patients: EGF, fractalkine, GRO, IL-4, IL-8, IP-10, MCP-1, MDC, PDGF-AA, sIL-2Ra, TGF-alpha, VEGF.
Full table
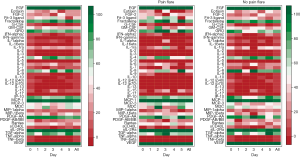
Comparing patients with or without pain flare EGF, fractalkine, GRO, IL-8, IP-10, MCP-1, MDC, sIL-2Ra, and TGF-alpha increased following radiation in both groups. Figures 3 and 4 show boxplot examples of changes in urine selected cytokines/chemokines post radiation in patients with or without pain flare. Pain flare patients had significant lower IL-8 levels (P=0.0007), IP-10 levels (P=0.046), and MDC levels (P=0.03) compared to non-pain flare patients. There was no significant time trends of these cytokines in patients with or without pain flare. For other cytokine variables, there was no significant time trends in all patients, or by pain flare. In general, all cytokine levels were stable over time.
Discussion
Short course radiotherapy can be used for palliating painful bone metastases. The exact mechanism of its palliative benefit is still unclear (9). Pain flare is a common side effect following palliative radiotherapy (10). This pain flare episode can be worrisome and debilitating for patients. Loblaw et al. compared a single 8 Gy fraction to 20 Gy in 5 fractions for the treatment of painful bone metastases. Their study concluded that patients receiving single fraction radiotherapy may be at a higher risk of pain flare (11). A single dose of dexamethasone can decrease the incidence of pain flare during the first 2 days immediately after palliative radiotherapy for bone metastases (12). They found in their phase II study that the incidence of pain flare dropped to 22% with the use of dexamethasone. The mechanism behind this phenomenon is still unkown but may be related to suppressing inflammatory cytokines.
Cytokines play a significant role in pain initiation and maintenance. Cytokines may be either pro or anti inflammatory, and an imbalance between the two is thought to contribute to pain flare (13). IL-8 (neutrophil chemotactic factor) cytokine is produced by macrophages and other cell types including epithelial cells. It acts as a signal attracting neutrophils to sites of inflammation (14). IP-10 cytokine is expressed by macrophages, neutrophils and epithelial cells (15). It has neutrophil chemo-attractant properties. It is involved in the processes of angiogenesis, inflammation, wound healing, and tumorigenesis. MDC cytokine recruits monocytes, memory T cells, and dendritic cells to sites of tissue injury, infection, and inflammation (16).
In conclusion, we demonstrated measurable changes in urinary cytokine/chemokine levels following radiation for painful bone metastases. Patients who experienced pain flare appear to have a different pattern in urinary cytokine/chemokine levels than patients without pain flare. To our knowledge, this is the first study that looked at the pathophysiology of pain flare through assessment of changes in urinary cytokines/chemokines in patients receiving EBRT for painful bone metastases to better understand the mechanism of pain flare. Our study was limited by small sample size. Further research is required to confirm the possible role of cytokines/chemokines in predisposing to and/or the cause of pain flare following radiation to painful bone metastases.
Acknowledgements
This study was funded by the research grant from the International Society of Oncology Pharmacy Practioners.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Presutti R, Hird A, DeAngelis C, et al. Palliative radiotherapy of bone metastases and pain flare. J Pain Management 2011;4:105-15.
- Lutz S, Berk L, Chang E, et al. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys 2011;79:965-76. [Crossref] [PubMed]
- Chow E, Zeng L, Salvo N, et al. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin Oncol (R Coll Radiol) 2012;24:112-24. [Crossref] [PubMed]
- Hird A, Chow E, Zhang L, et al. Determining the incidence of pain flare following palliative radiotherapy for symptomatic bone metastases: results from three canadian cancer centers. Int J Radiat Oncol Biol Phys 2009;75:193-7. [Crossref] [PubMed]
- Silberstein EB. Teletherapy and radiopharmaceutical therapy of painful bone metastases. Semin Nucl Med 2005;35:152-8. [Crossref] [PubMed]
- Sirera R, Salvador A, Roldán I, et al. Quantification of proinflammatory cytokines in the urine of congestive heart failure patients. Its relationship with plasma levels. Eur J Heart Fail 2003;5:27-31. [Crossref] [PubMed]
- Erickson DR, Xie SX, Bhavanandan VP, et al. A comparison of multiple urine markers for interstitial cystitis. J Urol 2002;167:2461-9. [Crossref] [PubMed]
- Chow E, Ling A, Davis L, et al. Pain flare following external beam radiotherapy and meaningful change in pain scores in the treatment of bone metastases. Radiother Oncol 2005;75:64-9. [Crossref] [PubMed]
- Chow E, Hird A, Zhang L, et al. Change in urinary markers of osteoclast activity following palliative radiotherapy for bone metastases. Clin Oncol (R Coll Radiol) 2009;21:336-42. [Crossref] [PubMed]
- Hird A, Wong R, Flynn C, et al. Impact of pain flare on patients treated with palliative radiotherapy for symptomatic bone metastases. J Pain Management 2009;4:401-6.
- Loblaw DA, Wu JS, Kirkbride P, et al. Pain flare in patients with bone metastases after palliative radiotherapy--a nested randomized control trial. Support Care Cancer 2007;15:451-5. [Crossref] [PubMed]
- Hird A, Zhang L, Holt T, et al. Dexamethasone for the prophylaxis of radiation-induced pain flare after palliative radiotherapy for symptomatic bone metastases: a phase II study. Clin Oncol (R Coll Radiol) 2009;21:329-35. [Crossref] [PubMed]
- McCune JS, Hawke RL, LeCluyse EL, et al. In vivo and in vitro induction of human cytochrome P4503A4 by dexamethasone. Clin Pharmacol Ther 2000;68:356-66. [Crossref] [PubMed]
- Hedges JC, Singer CA, Gerthoffer WT. Mitogen-activated protein kinases regulate cytokine gene expression in human airway myocytes. Am J Respir Cell Mol Biol 2000;23:86-94. [Crossref] [PubMed]
- Luster AD, Unkeless JC, Ravetch JV. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature 1985;315:672-6. [Crossref] [PubMed]
- Mantovani A, Gray PA, Van Damme J, et al. Macrophage-derived chemokine (MDC). J Leukoc Biol 2000;68:400-4. [PubMed]