
Hyperthyroidism due to thyrotropin receptor antibody stimulation of metastatic thyroid carcinoma during lenvatinib treatment: a case report
Introduction
Differentiated thyroid carcinoma (DTC) is the most common malignant tumor of the thyroid gland. Accordingly, radioactive iodine (RAI) therapy has been performed after total thyroidectomy for advanced differentiated carcinoma. When the tumor is refractory to RAI and progressive, multiple kinase inhibitors have been used for DTC. Phase III trials have shown that sorafenib and lenvatinib promoted longer progression-free survival than placebo in patients with RAI refracted DTC (1,2).
Although hyperthyroidism after total thyroidectomy is extremely rare, we had experienced a case of anti-thyrotropin receptor antibody (TRAb)-positive hyperthyroidism during lenvatinib treatment for metastatic DTC. Given that tumor progression was observed along with hyperthyroidism, TRAb stimulation may progress to advanced DTC. This has been the first case in which TRAb-positive hyperthyroidism occurred during multiple kinase inhibitor treatment for DTC. We present the following case in accordance with the CARE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-276/rc).
Case presentation
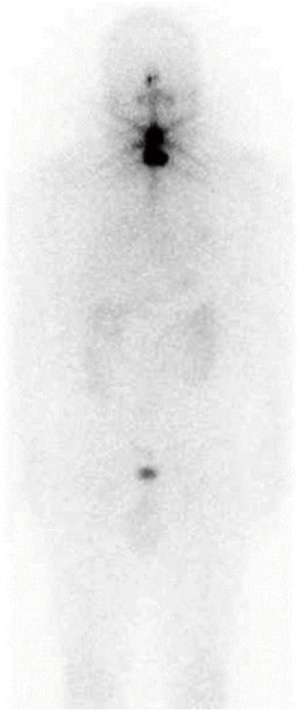
A 57-year-old man presented to our hospital with back pain and friction of the left hip. His family history included laryngeal cancer in his father and leukemia in his mother. His anamnesis revealed hypertension and follicular thyroid carcinoma (FTC) with lung metastasis. Six years ago, he underwent total thyroidectomy followed by RAI therapy for FTC with multiple lung metastases of less than 10 mm at another hospital. Blood tests before the surgery showed euthyroidism, a thyroglobulin (Tg) level of 4,920 ng/mL, and an anti-thyroglobulin antibody (TgAb) level of 31 IU/mL. The pathological diagnosis was FTC with partial capsular invasion with a maximum diameter of 65 mm. Despite administering 100 millicurie of iodine-131, scintigraphy showed no abnormal uptake except for the thyroid bed (Figure 1). Thereinafter, the patient stopped visiting the hospital.
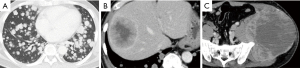
At the first visit to our hospital, computed tomography (CT) showed a 20-cm tumor at the left ilium and multiple metastases in the lung, liver, and bone (Figure 2). Blood tests revealed Tg of 322,000 ng/mL and TgAb of above 4,000 IU/mL. After left ilium biopsy, a pathological diagnosis of metastasis of thyroid carcinoma was established. After left iliac tumor decompression surgery, lenvatinib 20 mg/day perorally and denosumab 120 mg subcutaneous injection every 4 weeks were initiated.
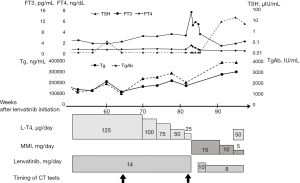
At 2 weeks after lenvatinib initiation, the patient needed 2 weeks of lenvatinib withdrawal due to diarrhea and anorexia. Lenvatinib was restarted at a dose of 14 mg/day. He discontinued treatment for 2 weeks due to lower limb edema at 8 weeks after treatment initiation. Thereafter, lenvatinib was continued at the dose of 14 mg. Tg and TgAb blood levels decreased to 88,600 ng/mL and 698 IU/mL, respectively. CT showed no progression of metastasis. Although thyrotropin (TSH) was suppressed at 125 µg of levothyroxine sodium (LT4), thyroid function was stable. However, free triiodothyronine (T3) blood levels started to increase at 70 weeks after lenvatinib initiation, and LT4 was gradually reduced to 25 µg. CT showed the progression of the lung and liver metastases 82 weeks after lenvatinib initiation (Figure 3, Table 1). At 83 weeks after lenvatinib initiation, the patient was hospitalized due to nausea, diarrhea, and anorexia. Blood free T3 and free thyroxine (T4) level increased to 13.98 pg/mL and 2.39 ng/dL, respectively. The blood concentrations of Tg and TgAb were 153,000 ng/mL and 1,870 IU/mL, respectively. There was no surge.

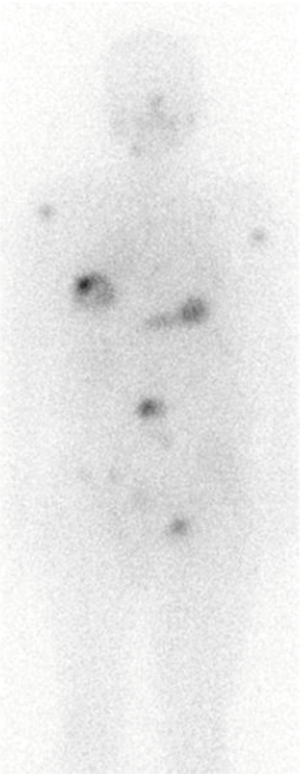
Table 1
| Timing of measurement | Site of metastasis | |
|---|---|---|
| Bone (mm3) | Liver (mm3) | |
| Before lenvatinib | 180×160×210 | 79×67×70 |
| Week 13 | 124×75×150 | 75×64×70 |
| Week 65 | 111×65×130 | 63×56×60 |
| Week 82 | 117×77×135 | 79×56×70 |
Admission examination revealed clear consciousness and a body temperature of 36.4 ℃, blood pressure of 118/63 mmHg, and heart rate of 104 beats/minute. Given that his blood anti-TRAb level was 28.3 IU/L (normal 0–1.9 IU/L), LT4 and lenvatinib were discontinued, and methimazole was administered at a dose of 15 mg/day. Lenvatinib was restarted 2 weeks after admission because the symptoms of hyperthyroidism had improved. Seven weeks after administration when the serum TSH level increased to 4.77 µIU/mL, iodine-131 scintigraphy of 3 millicurie was performed. It showed multiple uptakes of metastasis although the initial iodine scintigraphy showed no uptakes in lung metastasis (Figure 4). Considering that his thyroid function normalized, methimazole was gradually reduced to 5 mg/day (Figure 5). However, CT showed pleural effusion on both sides and tumor progression of the lung, liver, and adrenal metastases. The biopsy of the newly emerging skin metastases revealed a metastasis of thyroid carcinoma. Immunohistochemical staining indicated the presence of thyroid transcription factor 1 and Tg. There were no poorly differentiated or undifferentiated components. Anaplastic transformation was denied. He died of general prostration due to disease progression a year and 11 months after the initiation of lenvatinib.
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
DTC is the most common malignant tumor of the thyroid gland and includes papillary thyroid carcinoma and FTC. DTCs have TSH receptors, the stimulation of which promotes cell growth via the cyclic adenosine monophosphate (cAMP) cascade in thyroid cancer cells (3,4). A meta-analysis of clinical studies suggested that TSH suppression reduces the risks of major adverse clinical events in patients with DTC (5). Furthermore, a cohort study of 2,936 patients found that aggressive TSH suppression improved overall survival in high-risk patients (6). Given such findings, TSH suppression is recommended for high-risk DTCs (7).
Graves’ disease (GD) is an autoimmune disease known to be the most common cause of hyperthyroidism, the mechanism for which involves the binding of anti-TSH antibody binds to the TSH receptor. Typical blood test findings include decreased TSH levels and increased T3, T4, and TRAb levels (8). A recent meta-analysis reported that complication with GD was a risk factor for poor prognosis of DTC (9). There have been reports that several drugs can induce GD, including alemtuzumab, a CD52-targeting monoclonal antibody, and immune checkpoint inhibitors such as the human monoclonal antibody against cytotoxic T lymphocyte antigen-4 (CTLA4) (10,11). However, there have been no prior reports of lenvatinib causing GD.
Patients with advanced DTC hardly ever develop hyperthyroidism after total thyroidectomy. Although there are some case reports on GD complicated with thyroid cancer, most of them involved patients in their early stage of thyroid cancer or before treatment of thyroid cancer (12,13). In the last 10 years, only two cases of distant metastasis of thyroid carcinoma complicated with GD after total thyroidectomy have been reported (14,15). Before that, only six cases had been reported, with the study by Aoyama et al. summarizing them. The histological type was only FTC, and the metastatic site was lung and bone, both of which are predominant metastatic sites of DTC (14).
Lenvatinib is a multi-kinase inhibitor that suppresses intracellular signaling via the vascular endothelial growth factor (VEGF) receptors 1–3, fibroblast growth factor receptors 1–4, platelet-derived growth factor receptor-α, and the ret and KIT proto-oncogene receptor tyrosine kinases. Its main antitumor effect is by inhibiting angiogenesis (16). Thyroid cancer cell lines secrete more VEGF than normal thyrocytes, and the secretion of VEGF is increased by TSH. Notably, the expression of VEGF is correlated with the aggressiveness of thyroid cancers (17). Patients with GD have been found to have higher serum VEGF concentrations than healthy individuals. In addition, there is a correlation between the serum VEGF concentration and increased blood flow in the thyroid (18). The serum VEGF levels are increased in patients with active Graves’ ophthalmopathy (19). However, it is unlikely that lenvatinib induces GD because of its inhibitory effect toward VEGF signaling.
In the present case, RAI reuptake of bone metastasis was observed despite there being no accumulation at the time of the initial RAI. Although TKIs are used for RAI refractory DTC, RAI therapy can be performed after TKI therapy if thyroid tumor is unresectable at the start of treatment. Two prior cases of TKI therapy followed by RAI have been reported. One case was RAI naïve, and in the other case, accumulation was recognized by the previous RAI (20). However, these cases differ from our case, wherein there was no accumulation of metastasis at the time of the initial RAI. MAPK activation in BRAF or RAS mutant thyroid carcinoma silences the gene expression for iodide uptake and thyroid hormone biosynthesis. Some reports suggest that MAPK pathway inhibition with MEK or BRAF inhibitors can result in the reuptake of RAI in RAI refractory DTCs (21,22). However, there has been no report that lenvatinib can restore RAI accumulation in such cases. In our case, the recovered accumulation of RAI for lung metastases was not shown despite RAI reuptake for bone metastases. Although the reason for this is unclear, it may be due to the heterogeneity of the tumor or the influence of the environmental factors at the site of metastasis.
In a patient with FTC, GD, and Graves’ ophthalmopathy, the TSH receptor antagonist K1-70 decreased thyroid stimulating antibody activity and the Graves’ ophthalmopathy improved. In addition, FTC metastatic lesions stabilized during K1-70 monotherapy without lenvatinib (23). This case suggests that TSH receptor stimulation by TRAb can cause FTC progression and that a TSH receptor antagonist may benefit patients with FTC and GD.
In the present case, thyrotoxicosis and tumor progression occurred during lenvatinib treatment after total thyroidectomy. Destructive thyrotoxicosis is considered a cause of thyrotoxicosis. However, destructive thyrotoxicosis is not suitable for the present case because there was no rapid increase in the levels of Tg and the FT3/FT4 ratio was high. Moreover, iodine scintigraphy accumulation and TRAb positivity are atypical in thyrotoxicosis. Although there is no direct evidence that the metastases secreted thyroid hormone, the patient was thought to have developed hyperthyroidism mediated by TSH stimulation via TRAb. Notably, tumor progression was observed after the onset of hyperthyroidism. TRAb may have stimulated TSH receptors to cause tumor growth while hyperthyroidism suppressed TSH secretion. The differential diagnosis for the rapid growth of DTC was anaplastic transformation. However, skin biopsy ruled out anaplastic carcinoma when tumor regression and hyperthyroidism occurred. The candidate drugs for TSH receptor stimulated DTC are anti-thyroid drugs, immunosuppressive drugs, such as systemic glucocorticoids or rituximab, and TSH receptor antagonists, in accordance with Graves’ ophthalmopathy treatment. It took 13 weeks to diagnose GD since the patient’s thyroid function had started increasing. If the drugs reducing TRAb production had been initiated earlier to improve thyroid function and TRAb levels, tumor progression could have been slowed down.
Although multiple kinase inhibitors, such as lenvatinib or sorafenib, have been used for many cases of advanced DTC, which are often poorly differentiated, there are no reports of GD developing during treatment with multiple kinase inhibitors. Physicians suspecting hyperthyroidism during the treatment of advanced thyroid carcinoma should measure TRAb. In such cases, early interventions aimed at normalizing thyroid function and TRAb level should be considered.
In conclusion, this case report provides evidence that hyperthyroidism may occur in advanced thyroid cancer after total thyroidectomy requiring lenvatinib treatment. Persistent TSH stimulation caused by TRAb can be involved in tumor growth and thyroid hormone secretion from metastases.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-276/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-276/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet 2014;384:319-28. [Crossref] [PubMed]
- Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 2015;372:621-30. [Crossref] [PubMed]
- Ichikawa Y, Saito E, Abe Y, et al. Presence of TSH receptor in thyroid neoplasms. J Clin Endocrinol Metab 1976;42:395-8. [Crossref] [PubMed]
- Biondi B, Filetti S, Schlumberger M. Thyroid-hormone therapy and thyroid cancer: a reassessment. Nat Clin Pract Endocrinol Metab 2005;1:32-40. [Crossref] [PubMed]
- McGriff NJ, Csako G, Gourgiotis L, et al. Effects of thyroid hormone suppression therapy on adverse clinical outcomes in thyroid cancer. Ann Med 2002;34:554-64. [Crossref] [PubMed]
- Jonklaas J, Sarlis NJ, Litofsky D, et al. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid 2006;16:1229-42. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Smith TJ, Hegedüs L. Graves' Disease. N Engl J Med 2016;375:1552-65. [Crossref] [PubMed]
- Song Y, Fu L, Wang P, et al. Effect of Graves' disease on the prognosis of differentiated thyroid carcinoma: a meta-analysis. Endocrine 2020;67:516-25. [Crossref] [PubMed]
- Coles AJ, Wing M, Smith S, et al. Pulsed monoclonal antibody treatment and autoimmune thyroid disease in multiple sclerosis. Lancet 1999;354:1691-5. [Crossref] [PubMed]
- Sabini E, Sframeli A, Marinò M. A case of drug-induced Graves' Orbitopathy after combination therapy with Tremelimumab and Durvalumab. J Endocrinol Invest 2018;41:877-8. [Crossref] [PubMed]
- Folkestad L, Brandt F, Brix T, et al. Total Thyroidectomy for Thyroid Cancer Followed by Thyroid Storm due to Thyrotropin Receptor Antibody Stimulation of Metastatic Thyroid Tissue. Eur Thyroid J 2017;6:276-80. [Crossref] [PubMed]
- Suzuki K, Nakagawa O, Aizawa Y. A case of pulmonary metastatic thyroid cancer complicated with Graves' disease. Endocr J 2001;48:175-9. [Crossref] [PubMed]
- Aoyama M, Takizawa H, Tsuboi M, et al. A case of metastatic follicular thyroid carcinoma complicated with Graves' disease after total thyroidectomy. Endocr J 2017;64:1143-7. [Crossref] [PubMed]
- Abid SA, Stack BC Jr, Bodenner DL. Metastatic Follicular Thyroid Carcinoma Secreting Thyroid Hormone and Radioiodine Avid without Stimulation: A Case Report and Literature Review. Case Rep Endocrinol 2014;2014:584513. [Crossref] [PubMed]
- Matsui J, Yamamoto Y, Funahashi Y, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer 2008;122:664-71. [Crossref] [PubMed]
- Viglietto G, Maglione D, Rambaldi M, et al. Upregulation of vascular endothelial growth factor (VEGF) and downregulation of placenta growth factor (PlGF) associated with malignancy in human thyroid tumors and cell lines. Oncogene 1995;11:1569-79. [PubMed]
- Ramsden JD. Angiogenesis in the thyroid gland. J Endocrinol 2000;166:475-80. [Crossref] [PubMed]
- Ye X, Liu J, Wang Y, et al. Increased serum VEGF and b-FGF in Graves' ophthalmopathy. Graefes Arch Clin Exp Ophthalmol 2014;252:1639-44. [Crossref] [PubMed]
- Sheu NW, Jiang HJ, Wu CW, et al. Lenvatinib complementary with radioiodine therapy for patients with advanced differentiated thyroid carcinoma: case reports and literature review. World J Surg Oncol 2019;17:84. [Crossref] [PubMed]
- Iravani A, Solomon B, Pattison DA, et al. Mitogen-Activated Protein Kinase Pathway Inhibition for Redifferentiation of Radioiodine Refractory Differentiated Thyroid Cancer: An Evolving Protocol. Thyroid 2019;29:1634-45. [Crossref] [PubMed]
- Luckett KA, Cracchiolo JR, Krishnamoorthy GP, et al. Co-inhibition of SMAD and MAPK signaling enhances 124I uptake in BRAF-mutant thyroid cancers. Endocr Relat Cancer 2021;28:391-402. [Crossref] [PubMed]
- Ryder M, Wentworth M, Algeciras-Schimnich A, et al. Blocking the Thyrotropin Receptor with K1-70 in a Patient with Follicular Thyroid Cancer, Graves' Disease, and Graves' Ophthalmopathy. Thyroid 2021;31:1597-602. [Crossref] [PubMed]


