
Impact of radiotherapy for head and neck cancer on obstructive sleep apnea: a prospective study
Introduction
Obstructive sleep apnea (OSA) is a breathing disorder characterized by the repetitive collapse of the pharyngeal airway during sleep, leading to sleep fragmentation and oxygen desaturation. Risk factors for OSA in adults include aging, male gender, obesity, menopause, upper airway anatomy, and craniofacial abnormalities. Furthermore, OSA is an independent risk factor for hypertension, stroke, congestive heart failure, and coronary artery disease, and can thus induce significant morbidity and mortality (1-4).
The prevalence of upper airway tumors and cysts among patients complaining of snoring was 0.24% in a multi-center study (5). The prevalence of OSA with head and neck cancer (HNC) in the upper airway was reported to be 76% in oral cavity, oropharyngeal cancer and 72% in nasopharyngeal cancer (6,7). Because the head and neck areas are important for quality of life (QOL), by regulating functions such as swallowing and respiratory function, the definitive treatment of HNC requires a balance between cure and maintenance of QOL (8). The QOL of patients is considered a priority in the management and survival rate of HNC. For example, total laryngectomy or laryngopharyngectomy are standard procedures for advanced cancers of the larynx and/or hypopharynx, resulting in permanent tracheostomy and potential difficulties with speech and communication. Therefore, definitive radiation therapy (RT) alone, induction chemotherapy, and chemoradiotherapy (CRT) are considered necessary for organ preservation, and are mainstays of locally advanced HNC, but these combined radiation treatments can have serious side effects (9-11). We previously reported a case of severe OSA diagnosed and treated with continuous positive airway pressure (CPAP) after concurrent chemoradiotherapy (CRT) for laryngeal and hypopharyngeal cancer (12). Some authors have previously reported a relatively high prevalence of OSA in patients following RT for HNC, but little is known about the impact of such treatment on sleep disorders and their underlying mechanisms (13,14). To the best of our knowledge, most relevant studies to date have assessed only post-treatment OSA incidence (7,13,15-18). Furthermore, whether there is an association between RT-induced changes in pharyngeal morphology and OSA prevalence remains unknown.
The purpose of this prospective study was to elucidate the pathogenesis of OSA by comparing clinical and sleep test parameters and magnetic resonance imaging (MRI) findings before and after HNC treatment with radiation. The following article was performed in accordance with the STROBE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-267/rc).
Methods
Patients
A prospective study was conducted on consecutive patients diagnosed with HNC at Juntendo University Hospital or Juntendo Urayasu Hospital between May 2017 and August 2020.
Patients with nasopharyngeal, oropharyngeal, hypopharyngeal, and laryngeal cancers were included in the study. Definitive treatments included RT alone, CRT, bioradiotherapy (BRT), and super-selective intra-arterial concurrent chemoradiotherapy (IA-CRT). Almost all of the patients were treated with a radiation dose of 70 Gy in 35 fractions, except one who underwent IA-CRT with a radiation dose of 60 Gy in 30 fractions. The dose was fractionated into 2 Gy per day (five days/week). The exclusion criteria were as follows: (I) age <20 years; (II) treatment for sleep-disordered breathing, including use of oral appliances, CPAP therapy, and upper airway surgery prior to the sleep study; (III) history of surgery for HNC and/or planned adjuvant therapy; (IV) history of severe basic lung disease or neuromuscular disease; (V) presence of unknown untreated neoplasms other than HNC; and (VI) presence of distant metastases.
The Sleepiness Scale (ESS) and Pittsburgh Sleep Quality Index (PSQI) were applied both before and after treatment to evaluate subjective symptoms (19,20). Excessive daytime sleepiness was measured using the ESS, and scores of 11 and above were considered excessive daytime sleepiness. To measure subjective sleep quality, the PSQI, a self-administered questionnaire widely used to evaluate sleep quality, was also applied during the previous month.
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and was approved by the Ethics Committee of Juntendo University Faculty of Medicine (# 17-004). Informed consent was obtained from all patients.
Sleep study
The patients underwent overnight polysomnography (PSG) (Alice PDX; Philips Respironics, USA) or a portable sleep-testing device [WatchPat 200 (WP); Itamar Medical Ltd., Israel], supervised by sleep specialists, before and after treatment with HNC. First, all patients were suggested to undergo PSG. WP was performed when the patient did not consent to PSG. Each patient had the same device during the sleep tests, both before and after treatment. Pre-treatment sleep tests, both PSG and WP, were performed on the day before the first day of treatment in our otolaryngology, head, and neck ward. For post-treatment tests, only those who gave consent and had a sleep test during hospitalization were included. Based on our past experience, post-treatment tests were performed 2 months after starting the treatment (12). The results of the sleep test, including apnea-hypopnea index (AHI), lowest oxygen saturation (SO2), the time of SO2 less than 90% during sleep (SO2 <90%), and oxygen desaturation index (ODI), were evaluated. AHI was defined as the total number of apneas and hypopneas per hour of sleep, while OSA was defined as an AHI greater than five events per hour of sleep, of which ≥50% were obstructive in nature. The severity of OSA was determined as follows: 5≤ AHI <15, mild; 15≤ AHI <30, moderate; and 30≤ AHI as severe (21,22). The lowest SO2 and the time during which SO2 <90% were determined using a pulseoxymeter. ODI was defined as 4%ODI, as WP is scored at 4%ODI according to the rules of American Academy of Sleep Medicine by automatic analysis, and the same as PSG in order to match the conditions (23). The PSG analysis was performed by experienced technicians according to the AASM standard scoring rules (23). For WP data, AHI, lowest SO2, the time of SO2 <90%, and 4%ODI were analyzed automatically (zzzPAT ver.4.4.65.3). The 4%ODI is defined as a decrease in oxygen saturation exceeding 4% from the preceding baseline, and is the total number of oxygen desaturation events divided by the total monitoring time in hours. The interaction between AHI in PSG and WP was examined using a univariate general linear model (IBM SPSS Statistics; SPSS Inc., USA).
Cephalometric study
A lateral cephalometric radiograph was obtained in the upright position with natural head posture as part of the routine clinical evaluation (DRX-61A; Toshiba, Japan). The exposure parameters were arranged to clearly visualize bony landmarks before processing using an image-processing program (ImageJ; National Institutes of Health, USA). Blind analyses were performed by a single investigator (N.S.). As illustrated in Figure 1, the following seven cephalometric variables were measured to map craniofacial skeletal and soft tissue morphology, as described in previous reports (24-26).
MRI
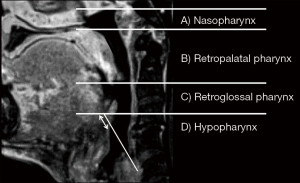
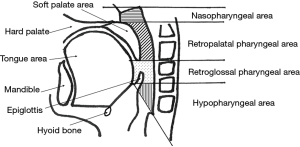
The laryngopharyngeal structures of the patients were evaluated using MRI in the midsagittal plane, before and after treatment. 3D T1-weighted images were acquired using a 3D turbo-field echo sequence, post-contrast with fat-saturation [repetition time (TR)/echo time (TE), 4.6–6.5/1.96–2.02 ms; flip angle, 15°; field of view (FOV), 300 mm], with a 1-mm thickness. A midline image in the sagittal plane was used for measurement. MRI examinations were performed before the start of treatment and two months after the end of RT. During scanning, the patients were placed in a supine position with their heads fixed in the neutral position in a holding frame, maintained calm breathing, and did not swallow. The blind analysis was performed by a single investigator. Each measurement was performed five times, and the median of the five measurements was used for the final analyses. We mapped the morphology of the pharyngeal structure using slight modifications of previously defined techniques (Figure 2) (27-29).
Statistical analysis
All statistical analyses were performed using EZR (The R Foundation for Statistical Computing, Austria) (30). Paired t-test was used for normally distributed continuous variables, and the Wilcoxon signed-rank test was used for non-normally distributed continuous variables. A value of P<0.05 was considered statistically significant. All values are shown as mean ± SD or median (interquartile range), unless indicated otherwise. No missing data were observed in this study.
Results
Overall, 32 patients with HNC were included. Of these, 21 patients consented to post-tests (2 underwent RT only, 17 underwent CRT, 1 underwent BRT, and 1 underwent IA-CRT). The 11 patients who declined to undergo post-treatment sleep tests did so for the following reasons: (I) the first sleep test was more painful and uncomfortable than expected; and (II) severe side effects of radiation therapy, such as sore throat and dermatitis. The AHI interaction between PSG and WP was not significant (P=0.949), indicating that AHI did not differ according to the sleep test device.
Overall, 32 patients underwent pre-treatment sleep tests to assess potential SDB. The clinical characteristics of the patients and the subjective and objective data before treatment are shown in Table 1. The prevalence of OSA in pre-treatment HNC was 81.3% (26 patients) and the mean AHI was 20.8±19.0 events/hr (AHI in PSG was 28.2±21.9, AHI in WP was 11.3±7.7).
Table 1
| Variable | Value |
|---|---|
| Age, years | 64.9±11.8 |
| Sex | Male: 31 (96.9%), female: 1 (3.1%) |
| Medical history, No. (%) | 13 (40.5) |
| Hypertension | 13 (40.5) |
| Diabetes mellitus | 7 (21.9) |
| Heart disease | 4 (12.5) |
| Lung disease | 2 (6.3) |
| Tumor location, No. (%) | |
| Nasopharynx | 7 (21.9) |
| Oropharynx | 4 (12.5) |
| Hypopharynx | 14 (43.8) |
| Larynx | 7 (21.9) |
| BMI, kg/m2 | 22.7±3.6 |
| Neck circumference, cm | 34.4±2.6 |
| Sleep data | |
| OSA (AHI ≥ 5), No. (%) | 26 (81.3) |
| AHI, events/h | 20.8±19.0 |
| Minimum SO2, % | 86.8±6.5 |
| SO2 <90%, min | 6.6±15.7 |
| ESS score | 5.2±3.4 |
| PSQI score | 6.3±4.0 |
N=32. Values are expressed as mean ± SD or number (percentage). BMI, body mass index; OSA, obstructive sleep apnea; AHI, apnea hypopnea index; SO2, oxygen saturation; ESS, Epworth sleepiness scale; PSQI, Pittsburgh Sleep Quality Index.
Later, 21 patients with HNC underwent a sleep test before and after treatment (Table 2). Among them, two underwent RT only, 17 underwent CRT, one underwent BRT, and one underwent IA-CRT. Although almost all patients had a complete response (CR) on post-treatment evaluation, one patient with nasopharyngeal cancer (cT4N2M0) had progressive disease (PD) with multiple metastases and had additionally started a molecular targeted drug. For subjective symptoms, there were no significant differences in the ESS (P=0.142) and PSQI (P=0.935) before and after treatment. BMI (P<0.0001) and neck circumference (P=0.0001) significantly decreased after treatment.
Table 2
| Variable | Sleep test | Pre-treatment | Post-treatment | P value |
|---|---|---|---|---|
| Age, years | 62.2±12.7 | |||
| Sex | Male: 20, female: 1 | |||
| Tumor location, No. (%) | ||||
| Nasopharynx | 7 (33.3) | |||
| Oropharynx | 4 (19.0) | |||
| Hypopharynx | 6 (28.6) | |||
| Larynx | 4 (19.0) | |||
| BMI, kg/m2 | All (N=21) | 22.4±4.0 | 20.2±3.4 | <0.0001 |
| PSG (N=9) | 23.2±5.1 | 20.9±4.6 | 0.0028 | |
| WP (N=12) | 21.9±3.3 | 19.7±2.3 | 0.0001 | |
| Neck circumference, cm | All (N=21) | 38.1±2.6 | 36.8±2.7 | 0.0001 |
| PSG (N=9) | 37.9±2.9 | 36.5±3.2 | 0.00997 | |
| WP (N=12) | 38.3±2.5 | 37.1±2.2 | 0.0068 | |
| ESS score | All (N=21) | 5.6±3.4 | 6.7±3.7 | 0.142 |
| PSG (N=9) | 6.1±4.2 | 7.3±4.9 | 0.217 | |
| WP (N=12) | 5.0±2.5 | 6.2±2.6 | 0.304 | |
| PSQI score | All (N=21) | 6.0±4.1 | 6.3±3.8 | 0.935 |
| PSG (N=9) | 7.3±4.3 | 6.5±3.5 | 0.436 | |
| WP (N=12) | 4.5±3.5 | 6.2±4.2 | 0.178 | |
N=21. Values are expressed as mean ± SD or number (percentage). PSG, polysomnography; WP, WatchPAT200; BMI, body mass index; ESS, Epworth sleepiness scale; PSQI, Pittsburgh Sleep Quality Index.
The sleep data of the pre- and post-treatment are presented in Table 3. The incidence of OSA in these patients was 81.0% (17 patients) before treatment and 85.7% (18 patients) after treatment (P=1.0). Regarding changes in the severity of OSA after treatment of HNC, patients with mild OSA increased from 33.3% to 38.1%, those with moderate OSA decreased from 28.6% to 19.0%, and those with severe OSA increased from 19.0% to 28.6%. There was no significant difference in OSA severity before and after treatment (P=0.064). AHI was increased in 57.1% of the patients (12 patients of the 21 patients, 4 with nasopharyngeal cancer, 1 with oropharyngeal cancer, 5 with hypopharyngeal cancer, 2 with laryngeal cancer) (P=0.327). Overall, the AHI was not significantly altered (from 14.5 to 14.9) after treatment (P=0.147). Similar results of AHI, 4%ODI, and minimum SO2 were obtained for each device. Severe OSA after treatment with CPAP indicated that two patients had accepted CPAP.
Table 3
| Variable | Sleep test | Pre-treatment, No. (%) | Post-treatment, No. (%) | P value |
|---|---|---|---|---|
| Severity of OSA | 17 (81.0) | 18 (85.7) | 1.0 | |
| Non OSA | 4 (19.0) | 3 (14.3) | 0.064 | |
| Mild to moderate | 13 (61.9) | 12 (57.1) | ||
| Severe | 4 (19.0) | 6 (28.6) | ||
| AHI, events/h | All (N=21) | 14.5 (17.3) | 14.9 (31.4) | 0.147 |
| PSG (N=9) | 21.2 (36.0) | 20.6 (60.2) | 0.359 | |
| WP (N=12) | 10.8 (12.2) | 14.5 (21.2) | 0.380 | |
| 4%ODI, events/h | All (N=21) | 5.0 (10.4) | 4.8 (19.8) | 0.191 |
| PSG (N=9) | 5.9 (19.1) | 9.8 (35.6) | 0.359 | |
| WP (N=12) | 4.1 (7.4) | 4.4 (11.7) | 0.410 | |
| Minimum SO2, % | All (N=21) | 89.0 (7.0) | 86.0 (8.5) | 0.162 |
| PSG (N=9) | 85.0 (12.0) | 85.0 (12.0) | 0.799 | |
| WP (N=12) | 90.0 (6.5) | 86.5 (5.5) | 0.13 | |
| SO2 <90%, min | All (N=21) | 0.1 (6.4) | 0.4 (7.3) | 0.398 |
| PSG (N=9) | 1.4 (33.9) | 0.8 (13.1) | 1.0 | |
| WP (N=12) | 0.0 (1.3) | 0.2 (2.1) | 0.202 |
N=21. Values are expressed as number (percentage). OSA, obstructive sleep apnea; PSG, polysomnography; WP, WatchPAT200; AHI, apnea hypopnea index; ODI, oxygen desaturation index; SO2, oxygen saturation.
Cephalometric parameters are listed in Table 4. Cephalometric data showed results similar to those of previous reports on skeletal morphology in the general Japanese population (26,31).
Table 4
| Measurement | Mean ± SD |
|---|---|
| SNA, ° | 82.05±4.98 |
| SNB, ° | 77.64±3.34 |
| ANB, ° | 4.41±2.63 |
| Facial axis angle, ° | 88.05±3.7 |
| PNS-P, mm | 43.62±4.94 |
| MP-H, mm | 12.93±6.75 |
| IAS, mm | 10.92±3.46 |
SNA, angle formed between the line SN to point “A”; SNB, angle formed between the line SN to point “B”; ANB, difference between SNA and SNB; PNS-P, length of uvula; MP-H, distance from mandibular plane to hyoid bone; IAS, positive airway space.
The results of the MRI parameters are listed in Table 5. In the midsagittal plane, the retroglossal pharyngeal area significantly increased after treatment (P=0.007). There were no statistical differences in nasopharyngeal, retropalatal, hypopharyngeal, tongue, soft palate, or epiglottis widths. Although the difference was not statistically significant, the nasopharyngeal and retropalatal areas tended to increase, and the retroglossal pharyngeal area was substantially larger than the pharyngeal morphology before treatment. Furthermore, the hypopharyngeal area tended to be smaller (P=0.071), and the width of the epiglottis tended to increase (P=0.074), although this difference was not statistically significant.
Table 5
| Parameter | Pre-treatment | Post-treatment | P value |
|---|---|---|---|
| Nasopharyngeal area, mm2 | 161.05±64.70 | 198.60±58.85 | 0.102 |
| Retropalatal pharyngeal area, mm2 | 333.66±53.29 | 345.44±78.58 | 0.558 |
| Retroglossal pharyngeal area, mm2 | 225.80±144.38 | 274.42±141.20 | 0.007 |
| Hypopharyngeal area, mm2 | 125.65±18.75 | 113.53±21.50 | 0.071 |
| Tongue area, mm2 | 2,876.86±418.11 | 2,791.41±312.18 | 0.201 |
| Soft palate area, mm2 | 374.55±72.51 | 370.47±77.79 | 0.752 |
| Width of the epiglottis, mm | 6.95±1.99 | 8.03±1.99 | 0.074 |
Values are expressed as mean ± SD.
Discussion
The present study revealed several novel and interesting observations concerning the relationship between OSA and HNC patients treated with RT. First, we found that, in untreated patients with HNC, the presence of OSA was high (81.3%), while the severity of OSA showed a wide range (mean AHI 20.8±19.0 events/hr). Second, in RT-treated patients, despite a significant decrease in the BMI and neck circumference, the presence of OSA increased from 81.0% to 85.7%. Third, patients who underwent RT in MRI imaging, the nasopharyngeal and retropalatal areas tended to have expanded, especially the retroglossal pharyngeal area, which was significantly larger than the pharyngeal morphology before treatment. Furthermore, the hypopharyngeal area tended to be smaller and the width of the epiglottis tended to increase.
The prevalence of OSA in the general middle-aged population has been estimated to be 4% in men and 2% in women (4). Upper airway abnormalities, such as tumors in the head and neck region, are common risk factors for OSA in adults (4,32). OSA can induce significant morbidity and mortality, and has been associated with severe daytime hypersomnolence and cardiovascular complications, including systemic hypertension, cardiac arrhythmias, and automobile accidents (33,34). Among patients complaining of snoring, 2.4 in 1,000 were found to have upper airway tumors, or cysts such as nasopharyngeal cancer in a multi-center research (5). Further, OSA with cancer in the upper airway has been reported to occur in 76% (13 of 17) of patients with cancer in the oral cavity and oropharynx, and in 72% (13 of 18) of patients with nasopharyngeal cancer. This was diagnosed by PSG in pre-treated patients with HNC (6,7). In our study, we found that the prevalence of OSA was high, at 81.25% (26 of 32 patients) in pre-treatment patients with pharyngeal and/or laryngeal cancer, which agrees with previous reports. The identification of OSA prior to treatment for HNC may be an important factor in improving quality of life after cancer therapy.
Our results showed that post-treatment patients with HNC had a much higher incidence of OSA (67%) compared to the normal population of middle-aged adults (2–4%) (4,35). Concerning the prevalence of OSA after radiation therapy for HNC, Friedman reported that all patients who received radiation therapy for pharyngeal or laryngeal cancer had OSA (35). Huyett found that 16 patients with a history of RT for oropharyngeal or laryngeal cancer completed a home sleep test, and half of the patients had OSA (13). Comparing our results with those of the normal population, we noticed a higher percentage of OSA, as found in previous reports on post-RT patients.
Furthermore, our results suggest that residual OSA may persist despite the disappearance of upper airway tumors after treatment. Excluding our report, only one study has so far specifically addressed HNC with OSA before and after RT in nasopharyngeal carcinoma, which targeted patients with nasopharyngeal cancer (7). In their study, AHI did not change significantly after treatment. These results were consistent with our study, which enrolled patients with cancer in all areas of the pharynx and larynx. We found that AHI did not significantly change after treatment, although it worsened somewhat in 12 of 21 patients (57.1%), and improved slightly in 9 of 21 patients (42.9%).
RT, such as CRT, for advanced carcinoma of the pharynx and larynx can reduce the need for surgical procedures, and is becoming a standard therapy to enable larynx preservation in patients with HNC (9,36). However, the disadvantages of irradiation, such as dry mouth, mucositis with sores, and difficulty in swallowing should be considered (12). Increased BMI and neck circumference have been reported to be important risk factors for OSA in the general population, whereas sleep apnea and hypoxia tend to improve after weight loss (37,38). In contrast to general OSA patients, most of our patients were not obese before HNC treatment (BMI <30 in 96.9%). Furthermore, BMI and neck circumference after treatment were significantly decreased compared to the pre-treatment values. Sore throat due to RT and nausea due to chemotherapy can both be considered as causes of weight loss. Upper airway narrowing due to pharyngeal edema is also common in HNC patients treated with RT (39,40). Mucosal edema after RT is a major radiation-induced side effect, and is generally represented as a thickened epiglottis on MRI (41). The predominant factor in patients with worsening OSA after RT is assumed to be reduced function and control of the pharyngeal dilator muscle, which may affect upper airway compliance and resistance and predispose individuals to OSA (7,14,16,35). In addition to edema of the upper airway after CRT, xerostomia and dysphagia are known to have adverse effects (39,40). Piccin et al. reported that the site of upper airway obstruction after treatment with HNC was the level of the larynx (15). In our study, the MRI findings showed that the width of the epiglottis tended to increase, although not significantly. Furthermore, the area of the upper airway, especially the retroglossal pharyngeal region, which is generally the main site of obstruction in OSA, was significantly larger than that before pre-treatment (27,42). The cause of this significant increase in the area may include weight loss and a decrease in neck circumference. Craniofacial anatomy is another factor associated with OSA. Non-obese patients with OSA have more disadvantageous cephalometric findings related to upper airway obstruction than obese patients (43). Regarding the cephalometric measurements in our patients, our case suggests that adverse cephalometric findings are less likely to be associated with OSA etiology.
Considering the results of this, the reason that 85.7% of residual OSA in post-treatment despite having a wider pharyngeal morphology is considered by dry mouth, dysphagia, and laryngeal dysfunction with irradiation (12,39,40). Consequently, laryngeal function might have decreased, and residual OSA was revealed after treatment.
This study had several limitations. First, bias may have been introduced because of diversity in population heterogeneity, OSA risk factors, different tumor locations, and the pathophysiology of HNC, including T stage and N stage. Variability in radiation and chemotherapy regimens was also a potential confounder. Second, the sample size was small because of the difficulty in obtaining consent for sleep tests, especially PSG, due to physical and mental distress. For this reason, two types of sleep test were conducted. Third, the follow-up period was limited to two months, and the patients were evaluated only in the acute phase after treatment. This should also be considered in the late phase. Finally, the functional site of the obstruction is unclear. Swallowing videoendoscopy and/or videofluorography are therefore required.
To the best of our knowledge, the present study is the first to show the pathophysiology of OSA with cancer in all areas of the pharynx and larynx, which were evaluated both before and after treatment, including irradiation. However, since the management of OSA after RT is not mentioned in the standard care of the NCCN guidelines, management of OSA may be an important factor in improving the quality of life of patients with HNC. Routine screening for OSA is important to improve the QOL of patients with advanced HNC.
Conclusions
This study found a higher prevalence of OSA both before and after radiation combined therapy for HNC compared to the normal population, even though our patients were not obese. Furthermore, AHI did not change despite a significant decrease in BMI after treatment. MRI revealed no significant narrowing of the airway. Therefore, we suggest that patients with HNC should be assessed for OSA. Early identification and treatment of OSA are important for the comprehensive management of patients with HNC.
Acknowledgments
Funding: This study was funded by Teijin Pharma Limited and Grant-in-Aid for Scientific Research.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-267/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-267/dss
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-267/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-267/coif). AI reports receiving research funding from Teijin Pharma Limited and article processing charges from Grant-in-Aid for Scientific Research. N Sata reports receiving research funding from Teijin Pharma Limited. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and was approved by the Ethics Committee of Juntendo University Faculty of Medicine (# 17-004). Informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lee W, Nagubadi S, Kryger MH, et al. Epidemiology of Obstructive Sleep Apnea: a Population-based Perspective. Expert Rev Respir Med 2008;2:349-64. [Crossref] [PubMed]
- He J, Kryger MH, Zorick FJ, et al. Mortality and apnea index in obstructive sleep apnea. Experience in 385 male patients. Chest 1988;94:9-14. [Crossref] [PubMed]
- Fletcher EC. The relationship between systemic hypertension and obstructive sleep apnea: facts and theory. Am J Med 1995;98:118-28. [Crossref] [PubMed]
- Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328:1230-5. [Crossref] [PubMed]
- Suzuki M, Saigusa H, Chiba S, et al. Prevalence of upper airway tumors and cysts among patients who snore. Ann Otol Rhinol Laryngol 2007;116:842-6. [Crossref] [PubMed]
- Payne RJ, Hier MP, Kost KM, et al. High prevalence of obstructive sleep apnea among patients with head and neck cancer. J Otolaryngol 2005;34:304-11. [Crossref] [PubMed]
- Lin HC, Friedman M, Chang HW, et al. Impact of head and neck radiotherapy for patients with nasopharyngeal carcinoma on sleep-related breathing disorders. JAMA Otolaryngol Head Neck Surg 2014;140:1166-72. [Crossref] [PubMed]
- Metreau A, Louvel G, Godey B, et al. Long-term functional and quality of life evaluation after treatment for advanced pharyngolaryngeal carcinoma. Head Neck 2014;36:1604-10. [Crossref] [PubMed]
- Pfister DG, Spencer S, Adelstein D, et al. Head and Neck Cancers, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2020;18:873-98. [Crossref] [PubMed]
- Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991;21:109-22. [Crossref] [PubMed]
- Fu KK, Woodhouse RJ, Quivey JM, et al. The significance of laryngeal edema following radiotherapy of carcinoma of the vocal cord. Cancer 1982;49:655-8. [Crossref] [PubMed]
- Inoshita A, Matsumoto F, Ohba S, et al. Severe obstructive sleep apnea after concurrent chemoradiotherapy for laryngeal and hypopharyngeal cancer managed by CPAP. Auris Nasus Larynx 2021; [Epub ahead of print]. [PubMed]
- Huyett P, Kim S, Johnson JT, et al. Obstructive sleep apnea in the irradiated head and neck cancer patient. Laryngoscope 2017;127:2673-7. [Crossref] [PubMed]
- Faiz SA, Balachandran D, Hessel AC, et al. Sleep-related breathing disorders in patients with tumors in the head and neck region. Oncologist 2014;19:1200-6. [Crossref] [PubMed]
- Piccin O, Sorrenti G, Milano F. Two Cases of Severe Obstructive Sleep Apnea Induced by Neck Radiotherapy Treated with an Oral Device. J Maxillofac Oral Surg 2016;15:400-3. [Crossref] [PubMed]
- Loth A, Michel J, Giorgi R, et al. Prevalence of obstructive sleep apnoea syndrome following oropharyngeal cancer treatment: A prospective cohort study. Clin Otolaryngol 2017;42:1281-8. [Crossref] [PubMed]
- Qian W, Haight J, Poon I, et al. Sleep apnea in patients with oral cavity and oropharyngeal cancer after surgery and chemoradiation therapy. Otolaryngol Head Neck Surg 2010;143:248-52. [Crossref] [PubMed]
- Stern TP, Auckley D. Obstructive sleep apnea following treatment of head and neck cancer. Ear Nose Throat J 2007;86:101-3. [Crossref] [PubMed]
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14:540-5. [Crossref] [PubMed]
- Doi Y, Minowa M, Uchiyama M, et al. Psychometric assessment of subjective sleep quality using the Japanese version of the Pittsburgh Sleep Quality Index (PSQI-J) in psychiatric disordered and control subjects. Psychiatry Res 2000;97:165-72. [Crossref] [PubMed]
- Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 1999;22:667-89. [Crossref] [PubMed]
- Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2012;8:597-619. [Crossref] [PubMed]
- Darien I. American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. American Academy of Sleep Medicine 2014.
- Inoshita A, Kasai T, Takahashi M, et al. Craniofacial anatomical risk factors in men with obstructive sleep apnea and heart failure: a pilot study. Sleep Breath 2014;18:439-45. [Crossref] [PubMed]
- Sata N, Inoshita A, Suda S, et al. Clinical, polysomnographic, and cephalometric features of obstructive sleep apnea with AHI over 100. Sleep Breath 2021;25:1379-87. [Crossref] [PubMed]
- Ahsan A, Yamaki M, Hossain Z, et al. Craniofacial cephalometric analysis of Bangladeshi and Japanese adults with normal occlusion and balanced faces: A comparative study. J Orthod Sci 2013;2:7-15. [Crossref] [PubMed]
- Taranto Montemurro L, Kasai T. The upper airway in sleep-disordered breathing: UA in SDB. Minerva Med 2014;105:25-40. [PubMed]
- Schwab RJ, Pasirstein M, Pierson R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med 2003;168:522-30. [Crossref] [PubMed]
- Malhotra A, Huang Y, Fogel RB, et al. The male predisposition to pharyngeal collapse: importance of airway length. Am J Respir Crit Care Med 2002;166:1388-95. [Crossref] [PubMed]
- Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 2013;48:452-8. [Crossref] [PubMed]
- Engel G, Spolter BM. Cephalometric and visual norms for a Japanese population. Am J Orthod 1981;80:48-60. [Crossref] [PubMed]
- White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med 2005;172:1363-70. [Crossref] [PubMed]
- Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 2000;283:1829-36. [Crossref] [PubMed]
- Hung J, Whitford EG, Parsons RW, et al. Association of sleep apnoea with myocardial infarction in men. Lancet 1990;336:261-4. [Crossref] [PubMed]
- Friedman M, Landsberg R, Pryor S, et al. The occurrence of sleep-disordered breathing among patients with head and neck cancer. Laryngoscope 2001;111:1917-9. [Crossref] [PubMed]
- Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 2003;349:2091-8. [Crossref] [PubMed]
- Davies RJ, Stradling JR. The relationship between neck circumference, radiographic pharyngeal anatomy, and the obstructive sleep apnoea syndrome. Eur Respir J 1990;3:509-14. [PubMed]
- Peppard PE, Young T, Palta M, et al. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 2000;284:3015-21. [Crossref] [PubMed]
- Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol 2008;26:3582-9. [Crossref] [PubMed]
- Braam PM, Terhaard CH, Roesink JM, et al. Intensity-modulated radiotherapy significantly reduces xerostomia compared with conventional radiotherapy. Int J Radiat Oncol Biol Phys 2006;66:975-80. [Crossref] [PubMed]
- Nömayr A, Lell M, Sweeney R, et al. MRI appearance of radiation-induced changes of normal cervical tissues. Eur Radiol 2001;11:1807-17. [Crossref] [PubMed]
- Katsantonis GP, Moss K, Miyazaki S, et al. Determining the site of airway collapse in obstructive sleep apnea with airway pressure monitoring. Laryngoscope 1993;103:1126-31. [Crossref] [PubMed]
- Matsuo A, Inoue Y, Tsuiki S, et al. Clinical characteristics of Japanese patients with familial obstructive sleep apnoea syndrome. Respirology 2010;15:93-8. [Crossref] [PubMed]


