
Effects of early postoperative shower after cardiac surgery
Introduction
Surgical site infection (SSI), which defined as infections occurring up to 30 days after surgery, is one of the most common complications associated with cardiac surgery. It results in prolonged hospital stay, compromised quality of life, and increased morbidity and costs (1). Several risk factors have been reported and various interventions to prevent SSI have been used, including preoperative bathing, smoking cessation, glucose control, methicillin-resistant Staphylococcus aureus (MRSA) screening, bowel preparations, skin preparation, surgical hand scrub, surgical gowns and masks, and antibiotic prophylaxis. In addition, daily wound dressing is recommended postoperatively (2).
Guidelines suggest that early showering does not increase the incidence of SSI. However, despite the lack of evidence, surgeons generally advise patients to keep the wound dry until suture removal or longer.
Several studies have previously suggested that early postoperative showering is not associated with SSI (3-5). However, little has been reported regarding early showering after cardiac surgery (6). The aim of our study was to evaluate the impact of early postoperative showering on wound complications. We present the following article in accordance with the STROBE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-306/rc).
Methods
This prospective observational study included 100 patients who underwent cardiac surgery at our institution. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Bucheon Sejong Hospital institutional review board approved the study protocol prior to patient enrollment and data collection (IRB No. 2026; Approval Date: July 09, 2021). Written informed consent was obtained from all the patients. Patients were eligible for this study if they were 19 years of age or older and had undergone cardiac surgery. Exclusion criteria included prolonged intensive care unit stay (more than 4 days) due to serious complications after surgery, open wounds, active infection, or prolonged drains. A total of 103 patients were enrolled in this study. However, 3 patients whose shower dates were more than 10 days after surgery were excluded. Consequently, 100 patients were included in this study.
Operative procedures
All the patients underwent cardiac surgery. Median sternotomy was performed in 48 patients while a minimally invasive approach was performed in 52 patients. The incision types included 48 median sternotomies, 39 right thoracotomies, 12 left thoracotomies, and 1 partial sternotomy. Wound closure was performed in the standard manner. The fascia and subcutaneous layer were then closed with 1-0 and 3-0 Vicryl (Ethicon, Cincinnati, OH, USA), respectively. Skin closure was performed using Dermabond (Ethicon, Cincinnati, OH, USA) in 44 cases, Zip surgical skin closure device (ZipLine Medical, Campbell, CA, USA) in 6, stapler in 11, both stapler and Zip surgical skin closure device in 38, and Steri-strips (3M, Two Harbors, MN, USA) in 1.
Prophylactic antibiotics were administered 1 hour before skin incision with cefazolin 2 g intravenously. If surgery is lengthened more than 4 hours, additional cefazolin 1 g was administered intravenously. After the operation, cefazolin 1 g was administered intravenously every 8 hours until 24 hours after the surgery.
Postoperative shower and wound care
After drains and pacing wires were removed, patients were allowed to shower in tap water without soaking or rubbing the wounds. The wounds were dried using a clean towel, and were left open without any dressing until the stitches were removed. Wound complications were assessed by the operating surgeon before discharge, at the 1st outpatient department (OPD) visit (approximately 7 days after discharge), and at the 2nd OPD visit (approximately 30 days after discharge).
Patient satisfaction questionnaire
Questionnaires were constructed to assess patient awareness of postoperative showers and satisfaction with early showering. All the patients completed the questionnaire at the 1st OPD visit. The questionnaire consisted of 5 items, as follows: (I) expected initiation of showers after surgery; (II) reason why early showers may not be allowed after surgery; (III) inconvenience of delayed showers; (IV) satisfaction with early showers; and (V) impact of early showering in choosing a surgeon or hospital. Data analysis was performed by generating percentage results or average scores for the responses to each question. The questionnaires are included in the Appendix 1.
Study endpoints and follow-up
The primary endpoints were wound complications, including wound dehiscence and superficial and deep wound infections. The wounds were evaluated by the operating surgeon before discharge and at the 1st and 2nd OPD visits, and any wound complications were assessed. Secondary endpoints included mortality, major adverse cardiac and cerebrovascular events defined as the composite of death, myocardial infarction, cerebrovascular accident, or stroke, valve-related complications, low cardiac output, reoperation, and acute kidney injury. Patient satisfaction was also assessed using questionnaires obtained from the patients at the 1st OPD visit.
Statistical analysis
Descriptive statistics were obtained for the entire study population. Categorical variables were presented as numbers and percentages. Continuous variables were presented as mean and standard deviation.
Results
Table 1 presents the baseline patient characteristics. The mean age was 63.0±13.5 years. The study included 44 women (44%). Thirty-six patients (36%) had a body mass index (≥25 kg/m2) and were categorized as overweight and obese. Diabetes was observed in 23 patients (23%). Steroid use was noted in 2 patients (2%). The mean left ventricular ejection fraction (LVEF) was 56.9%±8.7%. Seven patients (7%) had previous cardiac surgery.
Table 1
| Variables | Values |
|---|---|
| Age, years, mean ± SD | 63.0±13.5 |
| Sex, Female, n (%) | 44 (44.0) |
| Body surface area, m2, mean ± SD | 1.7±0.2 |
| BMI, kg/m2 | 24.2±3.5 |
| Overweight (30> BMI ≥25 kg/m2), n (%) | 32 (32.0) |
| Obesity (BMI ≥30 kg/m2), n (%) | 4 (4.0) |
| Hypertension, n (%) | 52 (52.0) |
| Diabetes mellitus, n (%) | 23 (23.0) |
| Stroke, n (%) | 8 (8.0) |
| Chronic kidney disease, n (%) | 5 (5.0) |
| Chronic obstructive pulmonary disease, n (%) | 3 (3.0) |
| Steroid use, n (%) | 2 (2.0) |
| Left ventricular ejection fraction, % | 56.9±8.7 |
| Previous open heart surgery, n (%) | 7 (7.0) |
| Atrial fibrillation, n (%) | 32 (32.0) |
BMI, body mass index.
The types of operations were aortic valve replacement in 51 patients (51%), mitral valve repair in 26, mitral valve replacement in 16, tricuspid valve repair in 23, maze operation in 19, coronary artery bypass grafting in 11, minimally invasive coronary artery bypass grafting in 12, and septal myectomy in 12. Detailed operative data are presented in Table 2.
Table 2
| Variables | Values |
|---|---|
| Cardiopulmonary bypass time, min, mean ± SD | 125.7±53.3 |
| Aortic cross clamp time, min, mean ± SD | 90.9±39.7 |
| Sternotomy, n (%) | 48 (48.0) |
| Minimally invasive cardiac surgery, n (%) | 52 (52.0) |
| Right thoracotomy | 39 |
| Left thoracotomy | 12 |
| Partial sternotomy | 1 |
| Aortic valve replacement, n (%) | 51 (51.0) |
| Mitral valve repair, n (%) | 26 (26.0) |
| Mitral valve replacement, n (%) | 16 (16.0) |
| Tricuspid valve repair, n (%) | 23 (23.0) |
| Maze operation, n (%) | 19 (19.0) |
| Coronary artery bypass grafting, n (%) | 11 (11.0) |
| Minimally invasive direct coronary artery bypass, n (%) | 12 (12.0) |
| Septal myectomy, n (%) | 12 (12.0) |
| Aortic operation, n (%) | 6 (7.0) |
| Myxoma, n (%) | 3 (3.0) |
| Other, n (%) | 3 (3.0) |
Outcomes
Table 3 shows early postoperative mortality and morbidity rates. There was no 30-day mortality. One patient developed postoperative stroke, 1 developed pneumonia, 1 underwent reoperation for bleeding, and 3 underwent pacemaker insertions.
Table 3
| Variables | Values |
|---|---|
| 30-day mortality, n (%) | 0 (0.0) |
| Postoperative stroke, n (%) | 1 (1.0) |
| Respiratory complications, n (%) | 1 (1.0) |
| Bleeding requiring reoperation, n (%) | 1 (1.0) |
| Permanent pacemaker insertion, n (%) | 3 (3.0) |
| Wound dehiscence, n (%) | 0 (0.0) |
| Superficial sternal wound infection, n (%) | 0 (0.0) |
| Deep sternal wound infection, n (%) | 0 (0.0) |
The mean time from surgery to shower was 6.0±1.4 days. Patients who underwent surgery through a minimally invasive approach showed shorter time from surgery to shower than those who underwent surgery through a median sternotomy (5.5±1.3 vs. 6.5±1.4 days, P<0.001).
No wound dehiscence, superficial wound infection, or deep wound infection was observed. Sternal exploration was required in 1 patient for periaortic fluid collection.
Summary of the questionnaires
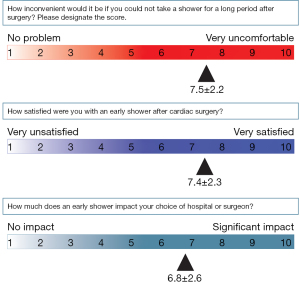
The answers to the questionnaires are summarized in Figures 1,2. For question 1 [When did you expect your possible shower date (including wounds) to be after cardiac surgery?], more than 50% of the patients thought that they could not shower until more than 2 weeks after the operation. For question 2 (Why did you think that you could not take early showers after surgery?), the main reason patients think they cannot take early showers was because “water contact may result in wound infection” (69%). For question 3 (How inconvenient would it be if you could not take a shower for a long period after surgery?), patients tended to feel uncomfortable with delayed showers, with a score of 7.5±2.2 out of 10. For question 4 (How satisfied were you with an early shower after cardiac surgery?), patients tended to be satisfied with early showers, with a score of 7.4±2.3 out of 10. For question 5 (How much does an early shower impact your choice of hospital or surgeon?), early showering was likely to affect the patient’s choice of hospital or surgeon, with a score of 6.8±2.6 out of 10.
Discussion
The main finding of this study was that early showers did not increase the risk of postoperative wound complications. No wound dehiscence, superficial wound infection, or deep wound infection was observed. The summary of the questionnaires showed a tendency toward high patient satisfaction for early showering after cardiac surgery.
Deep wound infection is a serious complication of cardiac surgery. If mediastinitis occurs, mortality increases up to 50% (7). Wound management is the basic element that prevents deep wound infection. Current guidelines suggest preoperative bathing, smoking cessation, glucose control, MRSA screening, bowel preparations, antibiotic prophylaxis, and wound care. The guidelines advise that the wound can be opened and showers may be taken 12 hours after surgery (2).
Several studies have also suggested that early showers after surgery do not increase postoperative wound complications. Hsieh et al. reported the results of a randomized study of 444 patients (222 showers vs. 222 non-showers) undergoing thyroid, lung, inguinal hernia, and face and extremity surgeries with clean or clean-contaminated wounds. They showed that superficial SSIs did not differ significantly between the 2 groups (8). Gök et al. conducted a randomized study of 51 patients (26 showers vs. 25 non-showers) who underwent coronary artery bypass grafting. Interestingly, the study showed that the rate of sternal wound infections was significantly lower in the shower group (P=0.083), suggesting that these findings might not be due to postoperative showering but due to surgery-related variables, especially a long hospital stay before surgery (6). A systematic review by Dayton et al. showed no increase in the incidence of infection in patients who were allowed to shower or bathe before suture removal compared to patients who kept the surgical site dry until suture removal (3).
However, there is a gap between currently available evidence and awareness in the clinical practice of wound care. Most surgeons and clinicians try to maintain wound dryness until suture removal for several reasons, such as lack of local evidence, lack of local clinical practice guidelines, difficulty in explaining to patients, and fear of legal problems. Furthermore, from the results of questionnaires in a previous study, most clinicians were not willing to allow water contact before the removal of stitches, even after they are made aware of the systematic review showing that the results of early postoperative showers after surgery were acceptable (9). Recently, Gök et al. reported a randomized study showing favorable results for early postoperative showers after coronary artery bypass grafting (6). However, 1 study may not be sufficient to change the actual practices of all cardiothoracic surgeons. Our study contributes to strengthening the evidence for early postoperative showering after cardiac surgery.
The results of our questionnaires also showed that more than half of the patients thought showers were allowed more than 2 weeks after surgery (Figure 1, Question 1). The most common reason was the belief that water contact may result in wound infection (Figure 1, Question 2). However, delayed showering after surgery was inconvenient for patients (7.5±2.2 out of 10, Figure 2), and patient satisfaction was high for early showers (7.4±2.3 out of 10, Figure 2). Patients also reported that early showers may affect the choice of hospital or surgeon (6.8±2.6 out of 10). In a previous study, the water-contact (early shower) group showed a significant increase in satisfaction compared with the control group (9). Early postoperative showers increase patient satisfaction without increasing postoperative wound complications.
In our study, 1 patient had a periaortic abscess requiring sternal exploration. A 47-year-old woman underwent aortic valve replacement, septal myectomy, and hemiarch replacement. Fever developed and persisted until postoperative day 5. Computed tomography (CT) showed a periaortic abscess. The patient underwent sternal exploration and abscess drainage. In addition, another 2 patients required sternal exploration for periaortic abscess; although the events in these 2 patients occurred after the last follow-up of the study (at the 2nd OPD visit, approximately 30 days after discharge), we are describing the patients due to their clinical significance. One patient was a 76-year-old woman who had undergone aortic valve replacement and septal myectomy. Wound discharge was noticed on postoperative day 143, and CT also revealed a periaortic abscess. The patient underwent sternal exploration and abscess drainage. The other patient was a 59-year-old woman who underwent aortic valve replacement. Wound swelling occurred at postoperative day 74. She also required sternal exploration and abscess drainage for periaortic abscess. Albumin/glutaraldehyde sealant (BioGlue, CryoLife, Kennesaw, GA, USA) was used in all 3 patients during the initial operation, and the development of a periaortic abscess was attributed to this. Several previous reports have described cold abscesses after the use of albumin/glutaraldehyde sealants (10,11). These sealants should be used cautiously.
The present study has several limitations. First, this was not a randomized controlled study and sample size calculation was not performed; therefore, we could not provide clear evidence of the clinical impact of postoperative showers. However, in our study, postoperative showers did not increase the risk of postoperative wound complications and enhanced patient satisfaction. Since only a few studies are available regarding postoperative showers after cardiac surgery, our findings are valuable for future randomized trials. Second, our study included a heterogeneous group of patients, using different surgical approaches. In a different context, this fact may also imply that postoperative showers can be universally applied, regardless of various surgical indications. Third, several different skin closure techniques were used in our study. Further study is necessary to evaluate the impacts of skin closure techniques on wound complications after cardiac surgery.
Conclusions
Our study suggests that early postoperative showers after cardiac surgery were not associated with an increased risk of wound complications, and resulted in high patient satisfaction. Early postoperative showering can be considered after cardiac surgery.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-306/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-306/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-306/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Bucheon Sejong Hospital institutional review board approved the study protocol prior to patient enrollment and data collection (IRB No. 2026; Approval Date: July 09, 2021). Written informed consent was obtained from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Badia JM, Casey AL, Petrosillo N, et al. Impact of surgical site infection on healthcare costs and patient outcomes: a systematic review in six European countries. J Hosp Infect 2017;96:1-15. [Crossref] [PubMed]
- Ban KA, Minei JP, Laronga C, et al. American College of Surgeons and Surgical Infection Society: Surgical Site Infection Guidelines, 2016 Update. J Am Coll Surg 2017;224:59-74. [Crossref] [PubMed]
- Dayton P, Feilmeier M, Sedberry S. Does postoperative showering or bathing of a surgical site increase the incidence of infection? A systematic review of the literature. J Foot Ankle Surg 2013;52:612-4. [Crossref] [PubMed]
- Feilmeier M, Dayton P, Sedberry S, et al. Incidence of surgical site infection in the foot and ankle with early exposure and showering of surgical sites: a prospective observation. J Foot Ankle Surg 2014;53:173-5. [Crossref] [PubMed]
- Heal C, Buettner P, Raasch B, et al. Can sutures get wet? Prospective randomised controlled trial of wound management in general practice. BMJ 2006;332:1053-6. [Crossref] [PubMed]
- Gök F, Demir Korkmaz F, Emrecan B. The effects of showering in 48-72 h after coronary artery bypass graft surgery through median sternotomy on wound infection, pain, comfort, and satisfaction: randomized controlled trial. Eur J Cardiovasc Nurs 2022;21:56-66. [Crossref] [PubMed]
- El Oakley RM, Wright JE. Postoperative mediastinitis: classification and management. Ann Thorac Surg 1996;61:1030-6. [Crossref] [PubMed]
- Hsieh PY, Chen KY, Chen HY, et al. Postoperative Showering for Clean and Clean-contaminated Wounds: A Prospective, Randomized Controlled Trial. Ann Surg 2016;263:931-6. [Crossref] [PubMed]
- Yu YH, Chao S, Lin YK, et al. The gap between currently available evidence and awareness in clinical practice of wound care: It is the time to shower earlier. Surgery 2018; Epub ahead of print. [Crossref]
- Yilmaz N, Sologashvili T, Huber C, et al. Sterile peri-graft abscess formation following aortic replacement: A word of caution for usage of BioGlue((R)). Int J Artif Organs 2021;44:917-9. [Crossref] [PubMed]
- Luk A, David TE, Butany J. Complications of Bioglue postsurgery for aortic dissections and aortic valve replacement. J Clin Pathol 2012;65:1008-12. [Crossref] [PubMed]



