
Unusual arm vein thrombosis after the Moderna (mRNA-1273) COVID-19 vaccination—a case report
Introduction
Uncountable number of reports regarding impact of coronavirus disease-2019 (COVID-19) on various fields of medicine has been published. However, exact association between this newly introduced virus and kinds of disease are under investigation since the all over the world are still suffering from dealing with severe form of acute respiratory syndrome. Among various adverse incidences related to COVID-19 infection itself or vaccination, venous thromboembolic events have been an issue. Occurrence of venous thromboembolic events after Moderna messenger ribonucleic acid (mRNA)-1273 COVID-19 seemed less common than those after mRNA vaccines of other manufacturers (1). For future study to evaluate exact association between COVID-19 vaccination and venous thromboembolism, we present one case of unusual arm vein thrombosis after mRNA-1273 COVID-19 vaccine. We present the following case in accordance with the CARE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-343/rc).
Case presentation
A 44-year-old Asian male visited outpatient clinic of vascular surgery department complaining of the right arm swelling. He developed right upper arm diffuse swelling and slightly bluish discoloration 4 days ago after receiving the third dose of mRNA-1273 COVID-19 vaccine in his left deltoid muscle. He stated that the left arm injection site discomfort such as mild swelling and pain was resolved, but the right arm swelling was much severe and getting hard to touch. Of note, he received all previous doses of vaccine from the same manufacturer over the left deltoid muscle since we usually choose non-dominant arm for injection site in Korea.
He was previously healthy with no comorbidities detected during routine health screening. He has no past medical and surgical history, and there was no remarkable family history including thrombotic disorders. However, he has been diagnosed COVID-19 based on positive polymerase chain reaction (PCR) test of nasopharyngeal swab 4 months before the third dose of mRNA-1273 COVID-19 vaccination. He received PCR test after close contact with his colleague confirmed positive PCR test. He was almost asymptomatic except mild cough, thus was released from 1-week quarantine without further disease progression.
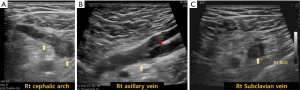
On physical examination, his right arm showed diffuse swelling with mild bluish discoloration and his right chest wall close to the right shoulder showed reticular veins. There were no abnormal blood laboratory tests except mild elevation of d-dimer level (4.2 µg/mL). Laboratory tests evaluating for thrombophilia [prothrombin time (PT), active partial thromboplastin time (aPTT), lupus anticoagulant, protein S activity, protein C activity, Anti-thrombin III, coagulation factor assay (both intrinsic and extrinsic), anti-cardiolipin antibody (IgG), and rheumatoid factor] were all negative. Venous duplex ultrasound revealed acute thrombosis in the right cephalic arch, axillary vein, and subclavian vein (Figure 1). To rule out venous type thoracic outlet syndrome, we evaluated if there was vein compression sign at the costo-clavicular space during the patient right arm was abducted using duplex ultrasonography (2). Since there was no structural abnormality, we did not perform further imaging studies.
Therapeutic oral anticoagulation was initiated with Rivaroxaban for a duration of 3 months. On his second visit 2 weeks after the initiation of anticoagulation, his right upper arm was still mildly hard to touch but swelling was relieved comparing to his arm at the first visit. Bluish discoloration was resolved, and chest wall reticular veins were almost disappeared. At follow-up visit upon completion of 3-month anticoagulation, venous duplex ultrasound revealed no remnant thrombosis. All procedures performed in this study were in accordance with the ethical standards of the institutional review board of Inha University Hospital and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
In Korea, AstraZeneca and Pfizer-BioNTech COVID-19 vaccines were initially introduced, and later Janssen and Moderna (mRNA-1273) COVID-19 vaccines were authorized to use (3). By October 31, 2021, 0.45% adverse events after any type of vaccination were reported among 78,416,802 doses in the country (3).
According to the report of Hwang et al., rate of adverse events was lowest after mRNA-1273 vaccine, but detailed types of adverse events were not described (3). In a recent American report, their adverse reaction collecting system had at least 17 cases of acute deep vein thrombosis (DVT) after mRNA-1273 vaccine, and those are mostly detected in the lower extremities (1).
There were several studies on association between COVID-19 mRNA vaccine and coagulopathy. Peyvandi et al. compared coagulation parameters in blood samples obtained from healthy individuals underwent Pfizer-BioNTech mRNA vaccination at pre-vaccination, 7 days and 21 days after the first dose, and 14 days after the second dose (4). They reported that coagulation parameters remained unaltered after vaccination except for a slight increase in platelet level (P=0.041). In another study of Campello et al., they also reported that there were no significant features of hypercoagulability or platelet activation after the first dose of BNT162b2 vaccination in healthy volunteers (5). Lim et al. also reported similar results after BNT162b2 vaccination showing no upregulated endothelial markers or hypercoagulability (6).
Since COVID-19 infection itself was known to be associated with arterial and venous thromboembolic events (7), we could not completely rule out his history of positive PCR from possible causes. Risk of venous thromboembolism after COVID-19 infection was studied by various investigators showing conflicting results from no increased risk (8) to higher risk than non-infected control group (9). Katsoularis et al. compared incidence rates of venous thromboembolism according to time period after COVID-19 infection (10). They reported significant increase in incidence rate ratio of DVT 70 days after COVID-19 infection. Given that his COVID-19 infection had been 4 months prior to his vaccination, and since patient had no prior co-morbidities or cardiovascular risk factors, we postulate that a possible cause of his right upper limb DVT may be secondary to his third dose of mRNA-1273 vaccine. However, understanding exact cause and/or interaction of mechanisms for venous thrombosis in our patient is incomplete. Another important limitation of this report is that exact thrombosis mechanism with Moderna (mRNA-1273) vaccine was not established since the previous studies were mostly done based on Pfizer mRNA vaccines. Despite the limitations, arm vein thrombosis at the contralateral arm to where the patient was vaccinated was a unique point to report. Further studies including comprehensive systematic reviews of the association of mRNA vaccination and thrombosis are needed. Clinicians should have a high index of suspicion for unusual cases of unilateral upper limb swelling post COVID-19 vaccination, and have a low threshold for evaluation for DVT.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The author has completed the CARE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-343/rc
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-343/coif). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional review board of Inha University Hospital and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bhan C, Bheesham N, Shakuntulla F, et al. An unusual presentation of acute deep vein thrombosis after the Moderna COVID-19 vaccine-a case report. Ann Transl Med 2021;9:1605. [Crossref] [PubMed]
- Baz AA. An overview of the findings of dynamic upper limbs’ arterial and venous duplex in cases of vascular thoracic outlet syndrome. Egypt J Radiol Nucl Med 2019;50:76. [Crossref]
- Hwang I, Park K, Kim TE, et al. COVID-19 vaccine safety monitoring in Republic of Korea from February 26, 2021 to October 31, 2021. Osong Public Health Res Perspect 2021;12:396-402. [Crossref] [PubMed]
- Peyvandi F, Scalambrino E, Clerici M, et al. No changes of parameters nor coagulation activation in healthy subjects vaccinated for SARS-Cov-2. Thrombosis Update 2021. doi:
10.1016/j.tru.2021.100059 .10.1016/j.tru.2021.100059 - Campello E, Simion C, Bulato C, et al. Absence of hypercoagulability after nCoV-19 vaccination: An observational pilot study. Thromb Res 2021;205:24-8. [Crossref] [PubMed]
- Lim XR, Leung BP, Sum CLL, et al. BNT162b2 mRNA SARS-CoV-2 vaccination does not cause upregulation of endothelial activation markers or hypercoagulability: A prospective, single-arm, longitudinal study. Am J Hematol 2022;97:E141-4. [Crossref] [PubMed]
- Martinez-Murillo C, Vargas-Ruiz AG. Coagulopathy, anticoagulant treatment in COVID-19 and thrombosis postvaccine. Gaceta médica de México 2021;157:S79-89.
- Mai V, Tan BK, Mainbourg S, et al. Venous thromboembolism in COVID-19 compared to non-COVID-19 cohorts: A systematic review with meta-analysis. Vascul Pharmacol 2021;139:106882. [Crossref] [PubMed]
- Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 2020;46:1089-98. [Crossref] [PubMed]
- Katsoularis I, Fonseca-Rodríguez O, Farrington P, et al. Risks of deep vein thrombosis, pulmonary embolism, and bleeding after covid-19: nationwide self-controlled cases series and matched cohort study. BMJ 2022;377:e069590. [Crossref] [PubMed]


