
Clinical efficacy of Mac-2-binding protein glycosylation isomer as a biomarker for albumin-bilirubin grade and the Controlling Nutritional Status score in chronic liver disease: investigation of cut-off values by the type of chronic liver disease
Introduction
Hepatic function reserve and nutritional status determine the prognosis for patients with chronic liver disease (CLD) (1-3). The Child-Turcotte classification was first published in 1964 as a prognostic criterion for cirrhosis and was used to classify patients receiving treatments such as transabdominal esophageal varicectomy or sclerotherapy. This classification was modified in 1973 by Pugh et al. as the Child-Pugh score (4). The major limitation of the Child-Pugh scoring system is that it includes several subjective parameters (hepatic encephalopathy and ascites) and interrelated parameters (ascites and serum albumin). Ascites can be easily influenced by diuretic use or dehydration state. Diagnosing minimal or covert hepatic encephalopathy involves difficulties. Hence, the albumin-bilirubin (ALBI) score uses only objective parameters, albumin (Alb) and total bilirubin (T. Bil), enabling a better evaluation. However, the limit of ALBI score is log calculation, so it is a little complicated in clinical use (5). In addition, the modified ALBI (mALBI) grade was proposed based on the relationship with indocyanine green values, and it has been utilized in clinical practice (6,7).
Nutritional status is also important for the assessment of CLD. The effect of immunological and nutritional status on the long-term prognosis of patients with CLD, including those with hepatocellular carcinoma, has been described. The effectiveness of the Controlling Nutritional Status (CONUT) score, which focuses on Alb, total lymphocyte count (TLC), and total cholesterol (TC) level, for systemic nutritional assessment, has been discussed by the nutrition support team (NST) and others (8). However, this tool is also cumbersome to use, requiring several items and the limit of CONUT score is leukocyte fraction, so it is affected by infectious diseases; thus, a single marker with a cut-off value for diagnosis is needed to examine the relationship with ALBI grade and CONUT score, and examine clinical application in hepatic reserve and nutritional status evaluation of patients with CLD.
In 2015, Mac-2-binding protein glycosylation isomer (M2BPGi), a carbohydrate antigen marker associated with fibrosis, was identified as a novel marker for liver fibrosis. M2BPGi is useful for the diagnosis of fibrosis in various liver diseases and changes in liver fibrosis. It has also been reported as a predictor of carcinogenesis in chronic hepatitis C and sustained virologic response of chronic hepatitis C (9).
Although M2BPGi is a biomarker of liver fibrosis, it is considered to have a function other than fibrosis because it is increased even in patients at high acute liver injury and inflammatory disease (10,11).
However, the relationship between M2BPGi and nutritional indices and assessments of hepatic function reserve in patients with CLD has not been investigated in detail. Thus, in this study, we aimed to evaluate the use of M2BPGi as a biomarker in patients with CLD to examine clinical application. We present the following article in accordance with the STROBE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-270/rc).
Methods
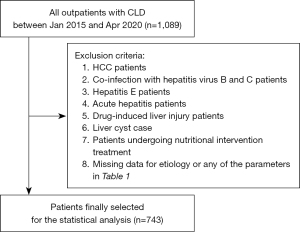
The relationship between the M2BPGi-CONUT score and M2BPGi-ALBI grade was investigated among 1,089 outpatients whose M2BPGi value was measured at their first visit to Saiseikai Niigata Hospital for CLD between January 2015 and April 2020. Seven hundred and forty-three patients whose CONUT score, ALBI grade, and M2BPGi were calculated from the same blood sample, and who did not meet any of the following exclusion criteria were included in the study (Figure 1). Patients with complications from hepatocellular carcinoma, those with missing data, and those in whom nutritional interventions such as branched-chain amino acids were already being used were excluded from the study.
ALBI grade was calculated using Alb and T. Bil as follows:
Patients with ALBI grade 1, 2 and 3 were allocated a score of 1, 2 and 3 points, respectively. Patients with mALBI grade 1, 2a, 2b and 3 were allocated a score of 1, 2 and 3 points, respectively.
The CONUT score was calculated using Alb, TLC and TC using the following formula:
where, serum albumin scores were 0: ≥3.5; 2: 3.0–3.49; 4: 2.50–2.99; 6: <2.50 g/dL; TC scores were 0: ≥180; 1: 140–179; 2: 100–139; 3: <100 mg/dL; and TLC scores were 0: ≥1.6109; 1: 1.20–1.59; 2: 0.80–1.19; 3: <0.8 g/L (8).
CONUT grade normal, light, moderate and severe were allocated of CONUT score.
FIB-4 was estimated using the following formula:
M2BPGi value was evaluated using HISCL M2BPGi Assay Kit with an automated immunoassay system HISCL-5000 (Sysmex, Hyogo, Japan), which takes 17 min and requires 10 µL sample of serum, allowing the results to be obtained on the date of blood sampling. M2BPGi levels were indexed using the following equation: cut-off index (COI) = (S-N)/(C-N), where S represents the light intensity in sample, N represents the light intensity in HISCL negative control and C represents the cut-off value.
The baseline characteristics of the 743 outpatients included in this study are shown in Table 1. The median age of patients was 67 (range, 59–75) years; 330 patients (44.41%) were males and 413 (55.59%) were females. CLD was related to hepatitis C virus (HCV) in 305 (41.0%) patients, hepatitis B virus (HBV) in 120 (16.2%) patients, nonalcoholic fatty liver disease/non-alcoholic steatohepatitis (NAFLD/NASH) in 167 (22.5%) patients, alcohol in 84 (11.3%) patients, and autoimmune hepatitis (AIH) in 67 (9.0%) patients. As the study was limited to patients with CLD under outpatient care, only six patients (0.81%) with ALBI grade 3 were included.
Table 1
| Parameter | Total (n=743) | HCV (n=305) | HBV (n=120) | NAFLD/NASH (n=167) | Alcoholic (n=84) | Autoimmune (n=67) |
|---|---|---|---|---|---|---|
| Age (years) | 67 [59–75] | 72 [65–80] | 61.5 [48–68] | 65 [56–74] | 65 [60–70] | 69 [63–76] |
| Sex, n (%) | ||||||
| Male | 330 (44.41) | 111 (36.39) | 69 (57.50) | 71 (42.51) | 65 (77.38) | 14 (20.90) |
| Female | 413 (55.59) | 194 (63.61) | 51 (42.50) | 96 (57.49) | 19 (22.62) | 53 (79.10) |
| AST (U/L) | 25 [20–32] | 23 [19–27] | 22 [19.25–27] | 30 [22–44] | 29 [22–44.5] | 26 [22–30] |
| ALT (U/L) | 17 [12–26] | 14 [11–20] | 16.5 [13–23] | 29 [17–49] | 22 [13.25–33] | 17 [13–22] |
| Serum albumin (g/dL) | 4.2 (3.9–4.4) | 4.2 (3.9–4.4) | 4.2 (4–4.38) | 4.2 (4–4.4) | 4.1 (3.6–4.4) | 4.1 (3.8–4.3) |
| Total bilirubin (mg/dL) | 0.59 (0.45–0.81) | 0.57 (0.43–0.76) | 0.74 (0.56–0.99) | 0.59 (0.46–0.79) | 0.57 (0.46–0.86) | 0.56 (0.42–0.67) |
| Prothrombin time (%) | 106.8 (96.8–115.7) | 107.7 (97.3–117.5) | 103.65 (92.85–114.88) | 109.9 (100.6–116.7) | 103.2 (89.65–110.18) | 105.8 [96–115] |
| Platelet count (×104/mL) | 19.3 (15.5–23.6) | 18.2 (15–22.25) | 19.85 (14.95–22.78) | 20.5 (17–24.4) | 18.65 (14.03–22.83) | 23.2 (19–30.5) |
| CONUT score | 1 [0–2] | 1 [0–2] | 2 [0–2] | 1 [0–2] | 1 [0–2] | 2 [1–3] |
| TC (mg/dL) | 185 [161–207] | 188 [165.5–212.5] | 185 [164–199] | 177 [157–197] | 182 [161–202] | 189 [168–211] |
| Lymphocyte count (%) | 15.8 (11.9–21.3) | 15.8 (12–21.7) | 13.65 (10.32–18.1) | 17.3 (14.7–23.3) | 15.95 (11.93–20.90) | 13.6 (9.9–19.1) |
| M2BPGi (COI) | 0.82 (0.57–1.32) | 0.95 (0.67–1.55) | 0.66 (0.47–1.03) | 0.69 (0.48–1.1) | 0.91 (0.68–1.61) | 0.93 (0.57–1.35) |
| Fib-4 index | 2.17 (1.43–3.09) | 2.4 (1.75–3.27) | 1.84 (1.03–2.84) | 1.88 (1.19–2.71) | 2.37 (1.72–3.77) | 1.8 (1.21–2.8) |
| ALBI score | −2.9 (−3.1, −2.7) | −2.9 (−3.1, −2.7) | −2.8 (−3.01, −2.6) | −2.9 (−3.1, −2.7) | −2.8 (−3.08, −2.45) | −2.9 (−3.1, −2.6) |
| mALBI grade, n (%) | ||||||
| 1 | 595 (80.08) | 251 (82.30) | 97 (80.83) | 142 (85.03) | 55 (65.48) | 50 (74.63) |
| 2a | 90 (12.11) | 35 (11.48) | 14 (11.67) | 14 (8.38) | 16 (19.05) | 11 (16.42) |
| 2b | 52 (7.00) | 19 (6.23) | 6 (5.00) | 10 (5.99) | 12 (14.29) | 5 (7.46) |
| 3 | 6 (0.81) | 0 | 3 (2.50) | 1 (0.60) | 1 (1.19) | 1 (1.49) |
Data are presented as n (%), or median (interquartile range). CLD, chronic liver disease; HCV, hepatitis C virus; HBV, hepatitis B virus; NAFLD/NASH, nonalcoholic fatty liver disease/non-alcoholic steatohepatitis; AST, aspartate aminotransferase; ALT, alanine aminotransferase; CONUT, Controlling Nutritional Status; TC, total cholesterol; M2BPGi, mac-2-binding protein glycosylation isomer; COI, cut-off index; ALBI, albumin-bilirubin; mALBI, modified albumin-bilirubin.
In this study, we determined the relationship between M2BPGi and nutritional status and hepatic function reserve in patients with CLD.
Ethics statement
The study was approved by the Institutional Review Board of Saiseikai Niigata Hospital (No. E18-18) and was performed in accordance with the principles of the Declaration of Helsinki (as revised in 2013). All patients provided written informed consent.
Statistical analysis
Data are expressed as median and interquartile range unless otherwise stated. Spearman’s rank correlations were performed to evaluate the serum M2BPGi with ALBI grade and other clinical features. Results with 0≤r<0.3 were considered almost irrelevant. Results with 0.3≤r<0.5 were considered almost a very weak correlation. Results with 0.5≤r<0.7 were considered almost a correlation. Results with 0.7≤r<0.9 were considered almost a strong correlation. Pairwise comparisons were made using Wilcoxon’s rank-sum test for between-group comparisons. To evaluate the diagnostic performance of M2BPGi in assessing hepatic function reserve and nutritional status, receiver operating characteristic (ROC) curve analysis was performed. Diagnostic accuracy is expressed as specificity, sensitivity, positive predictive value (PPV), negative ROC curve predictive value, and area under the ROC curve. The optimal cut-off values were obtained by maximizing Youden’s index (sensitivity + specificity-1). Results with P<0.05 were considered statistically significant. All statistical analyses were performed using JMP15.2.0 (SAS Institute Inc., Cary, NC, USA).
Results
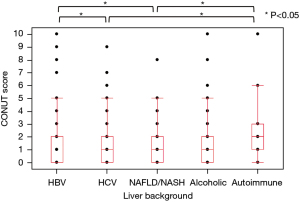
The distribution of M2BPGi, CONUT, and ALBI by the underlying liver disease type is shown in Figures 2-4, respectively. The pairwise Wilcoxon test revealed significant differences among some groups.
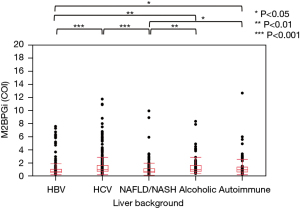
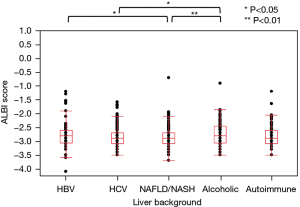
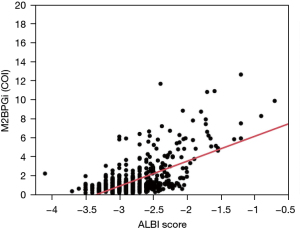
The correlation coefficient (r2) between M2BPGi and ALBI grade was 0.40 (r=0.63), indicating a positive correlation between M2BPGi and ALBI grade, where M2BPGi increased with ALBI and mALBI grade (Figure 5). The r2 between FIB-4 index and CONUT score was 0.17 (r=0.41), indicating a very weak positive correlation between the FIB-4 index and CONUT score.
The r2 between the FIB-4 index and ALBI grade was 0.22 (r=0.47), indicating a very weak positive correlation between FIB-4 index and ALBI score.
All cases (n=743) (Table 2)
Table 2
| Categories | Cut-off value | AUC | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | TP | TN | FP | FN |
|---|---|---|---|---|---|---|---|---|---|---|
| Total (n=743) | ||||||||||
| G1 vs. G2–G3 | 1.07 | 0.82 | 75.00 | 75.80 | 43.53 | 92.42 | 111 | 451 | 144 | 37 |
| G1–G2a vs. G2b–G3 | 1.73 | 0.89 | 77.59 | 86.42 | 32.61 | 97.85 | 45 | 592 | 93 | 13 |
| G1–G2 vs. G3 | 5.83 | 0.99 | 100.00 | 97.15 | 22.22 | 100.00 | 6 | 716 | 21 | 0 |
| HCV (n=305) | ||||||||||
| G1 vs. G2–G3 | 1.93 | 0.82 | 68.52 | 90.84 | 61.67 | 93.06 | 37 | 228 | 23 | 17 |
| G1–G2a vs. G2b–G3 | 1.78 | 0.86 | 78.95 | 82.17 | 22.73 | 98.33 | 15 | 235 | 51 | 4 |
| HBV (n=120) | ||||||||||
| G1 vs. G2–G3 | 1.30 | 0.83 | 73.91 | 93.81 | 73.91 | 93.81 | 17 | 91 | 6 | 6 |
| G1–G2a vs. G2b–G3 | 4.63 | 0.97 | 100.00 | 96.40 | 69.23 | 100.00 | 9 | 107 | 4 | 0 |
| G1–G2 vs. G3 | 5.83 | 0.98 | 100.00 | 96.58 | 42.86 | 100.00 | 3 | 113 | 4 | 0 |
| NAFLD/NASH (n=167) | ||||||||||
| G1 vs. G2–G3 | 0.68 | 0.82 | 92.00 | 55.63 | 26.74 | 97.53 | 23 | 79 | 63 | 2 |
| G1–G2a vs. G2b–G3 | 1.49 | 0.92 | 81.82 | 92.31 | 42.86 | 98.63 | 9 | 144 | 12 | 2 |
| G1–G2 vs. G3 | 9.86 | 1.00 | 100.00 | 100.00 | 100.00 | 100.00 | 1 | 166 | 0 | 0 |
| Alcoholic (n=84) | ||||||||||
| G1 vs. G2–G3 | 0.88 | 0.82 | 89.66 | 65.45 | 57.78 | 92.31 | 26 | 36 | 19 | 3 |
| G1–G2a vs. G2b–G3 | 2.80 | 0.88 | 69.23 | 98.59 | 90.00 | 94.59 | 9 | 70 | 1 | 4 |
| G1–G2 vs. G3 | 8.27 | 1.00 | 100.00 | 100.00 | 100.00 | 100.00 | 1 | 83 | 0 | 0 |
| Autoimmune (n=67) | ||||||||||
| G1 vs. G2–G3 | 1.08 | 0.87 | 88.24 | 78.00 | 57.69 | 95.12 | 15 | 39 | 11 | 2 |
| G1–G2a vs. G2b–G3 | 1.22 | 0.83 | 83.33 | 75.41 | 25.00 | 97.87 | 5 | 46 | 15 | 1 |
| G1–G2 vs. G3 | 12.63 | 1.00 | 100.00 | 100.00 | 100.00 | 100.00 | 1 | 66 | 0 | 0 |
M2BPGi, mac-2-binding protein glycosylation isomer; mALBI, modified albumin-bilirubin; AUC, area under the curve; PPV, positive predictive value; NPV, negative predictive value; TP, true positive; TN, true negative; FP, false positive; FN, false negative; G1, grade 1; G2, grade 2; G3, grade 3; HCV, hepatitis C virus; HBV, hepatitis B virus; NAFLD/NASH, nonalcoholic fatty liver disease/non-alcoholic steatohepatitis.
M2BPGi predicted mALBI G1 vs. G2–G3 with a sensitivity of 0.75 and specificity of 0.76 when area under the curve (AUC) =0.82, cut-off 1.07. M2BPGi predicted mALBI G1–2a vs. G2b–G3 with a sensitivity of 0.78 and specificity of 0.86 when AUC =0.89, cut-off 1.73. M2BPGi predicted mALBI G1–2 vs. G3 with a sensitivity of 1.00 and specificity of 0.97 when AUC =0.99, cut-off 5.83.
HCV (n=305)
There were no G3 cases among patients with HCV. M2BPGi predicted mALBI G1–G2a vs. G2b with a sensitivity of 0.79 and specificity of 0.82 when AUC =0.86, cut-off 1.78.
HBV (n=120)
M2BPGi predicted mALBI G1–2a vs. G2b–G3 with a sensitivity of 1.00 and specificity of 0.96 when AUC =0.97, cut-off 4.63. M2BPGi predicted mALBI G1–G2 vs. G3 with a sensitivity of 1.00 and specificity of 0.97 when AUC =0.98, cut-off 5.83.
NAFLD/NASH (n=167)
M2BPGi predicted mALBI G1 vs. G2–G3 with a sensitivity of 0.92 and specificity of 0.56 when AUC =0.82, cut-off 0.68. M2BPGi predicted mALBI G1–G2a vs. G2b–G3 with a sensitivity of 0.82 and specificity of 0.92 when AUC =0.92, cut-off 1.49. M2BPGi predicted mALBI G1–G2 vs. G3 with a sensitivity of 1.00 and specificity of 1.00 when AUC =1.00, cut-off 9.86.
Alcoholic liver disease (n=84)
M2BPGi predicted mALBI G1–G2 vs. G3, with a sensitivity of 1.00 and specificity 1.00 when AUC =1.00, cut-off 8.27.
AIH (n=67)
M2BPGi predicted mALBI G1–G2 vs. G3 with a sensitivity and specificity of 1.00 when AUC =1.00, cut-off 12.63.
Next, we analyzed the relationship between M2BPGi and CONUT scores (Table 3). M2BPGi predicted CONUT score normal vs. light-severe with a sensitivity of 0.38 and specificity of 0.92 when AUC =0.65, cut-off 1.60. M2BPGi predicted CONUT score normal-light vs. moderate-severe with a sensitivity of 0.91 and specificity of 0.85 when AUC =0.93, cut-off 1.74. M2BPGi predicted CONUT score normal-moderate vs. severe with a sensitivity of 1.00 and specificity of 0.97 when AUC =0.99, cut-off 5.83.
Table 3
| Categories | Cut-off value | AUC | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | TP | TN | FP | FN |
|---|---|---|---|---|---|---|---|---|---|---|
| Total (n=743) | ||||||||||
| Grade normal vs. light-severe | 1.60 | 0.65 | 38.41 | 92.07 | 75.51 | 70.13 | 111 | 418 | 36 | 178 |
| Grade normal-light vs. moderate-severe | 1.74 | 0.93 | 90.63 | 84.81 | 21.17 | 99.50 | 29 | 603 | 108 | 3 |
| Grade normal-moderate vs. severe | 5.83 | 0.99 | 100.00 | 97.15 | 22.22 | 100.00 | 6 | 716 | 21 | 0 |
| HCV (n=305) | ||||||||||
| Grade normal vs. light-severe | 1.60 | 0.71 | 49.06 | 88.44 | 69.33 | 76.52 | 52 | 176 | 23 | 54 |
| Grade normal-light vs. moderate-severe | 5.40 | 0.87 | 69.23 | 97.60 | 56.25 | 98.62 | 9 | 285 | 7 | 4 |
| Grade normal-moderate vs. severe | 10.89 | 1.00 | 100.00 | 99.67 | 50.00 | 100.00 | 1 | 303 | 1 | 0 |
| HBV (n=120) | ||||||||||
| Grade normal vs. light-severe | 1.30 | 0.66 | 36.07 | 98.31 | 95.65 | 59.79 | 22 | 58 | 1 | 39 |
| Grade normal-light vs. moderate-severe | 4.63 | 0.96 | 100.00 | 95.54 | 61.54 | 100.00 | 8 | 107 | 5 | 0 |
| Grade normal-moderate vs. severe | 5.83 | 0.98 | 100.00 | 96.58 | 42.86 | 100.00 | 3 | 113 | 4 | 0 |
| NAFLD/NASH (n=167) | ||||||||||
| Grade normal vs. light-severe | 1.73 | 0.60 | 26.32 | 98.18 | 88.24 | 72.00 | 15 | 108 | 2 | 42 |
| Grade normal-light vs. moderate-severe | 1.74 | 0.95 | 100.00 | 92.07 | 18.75 | 100.00 | 3 | 151 | 13 | 0 |
| Alcoholic (n=84) | ||||||||||
| Grade normal vs. light-severe | 2.79 | 0.60 | 31.25 | 98.08 | 90.91 | 69.86 | 10 | 51 | 1 | 22 |
| Grade normal-light vs. moderate-severe | 1.38 | 0.92 | 100.00 | 75.95 | 20.83 | 100.00 | 5 | 60 | 19 | 0 |
| CONUT grade normal-moderate vs. severe | 8.27 | 1.00 | 100.00 | 100.00 | 100.00 | 100.00 | 1 | 83 | 0 | 0 |
| Autoimmune (n=67) | ||||||||||
| Grade normal vs. light-severe | 1.08 | 0.69 | 57.58 | 79.41 | 73.08 | 65.85 | 19 | 27 | 7 | 14 |
| Grade normal-light vs. moderate-severe | 2.78 | 0.97 | 100.00 | 90.63 | 33.33 | 100.00 | 3 | 58 | 6 | 0 |
| Grade normal-moderate vs. severe | 12.63 | 1.00 | 100.00 | 100.00 | 100.00 | 100.00 | 1 | 66 | 0 | 0 |
M2BPGi, mac-2-binding protein glycosylation isomer; CONUT, Controlling Nutritional Status; AUC, area under the curve; PPV, positive predictive value; NPV, negative predictive value; TP, true positive; TN, true negative; FP, false positive; FN, false negative; HCV, hepatitis C virus; HBV, hepatitis B virus; NAFLD/NASH, nonalcoholic fatty liver disease/non-alcoholic steatohepatitis.
HCV (n=305) (CONUT score)
M2BPGi predicted CONUT score normal-moderate vs. severe with a sensitivity and specificity of 1.00 when AUC =1.00, cut-off 10.89.
HBV (n=120) (CONUT score)
M2BPGi predicted CONUT score normal-light vs. moderate-severe with a sensitivity of 1.00 and specificity of 0.96 when AUC =0.96, cut-off 4.63.
M2BPGi predicted CONUT score normal-moderate vs. severe with a sensitivity of 1.00 and specificity of 0.97 when AUC =0.98, cut-off 5.83.
None of the patients with NADH/NAFLD were evaluated as CONUT severe. M2BPGi predicted CONUT score normal-light vs. moderate with a sensitivity of 1.00 and specificity of 0.92 when AUC =0.95, cut-off 1.74.
Alcoholic liver disease (n=84) (CONUT score)
M2BPGi predicted CONUT score normal-light vs. moderate-severe with a sensitivity of 1.00 and specificity of 0.76 when AUC =0.92, cut-off 1.38.
AIH (n=67) (CONUT score)
M2BPGi predicted CONUT score normal-light vs. moderate-severe with a sensitivity of 1.00 and specificity of 0.91 when AUC =0.97, cut-off 2.78.
Discussion
Malnutrition is common in CLD, and early intervention is necessary to improve patient prognosis (12,13). Serum albumin is the main factor used to evaluate CLD and is included in both ALBI and Child-Pugh scores. The progression of CLD, including viral and non-viral diseases, involves cirrhosis and hepatocellular carcinoma as a consequence of liver fibrosis. Recently, HISCL M2BPGi, which allows the evaluation of progression of liver fibrosis via glycan markers in serum, has been developed; it is commercially available (14).
Various studies have evaluated the clinical relationship between M2BPGi and CLD pathogenesis (15-18).
Although M2BPGi has the potential for use as a biomarker during treatment and could be used to evaluate prognosis in clinical practice, clinical data are insufficient. In this study, we aimed to clarify the clinical usefulness of measuring the hepatic fibrosis marker M2BPGi at the first outpatient visit in individuals with CLD and to determine whether it would be suitable as a biomarker for nutritional status by ALBI and CONUT, which are indicators of hepatic function reserve.
M2BPGi, Wisteria floribunda agglutinin-positive Mac 2-binding protein (WFA+-M2BP), is a carbohydrate structure on M2BP that increases in concentration with the progression of liver disease. M2BPGi is a donut-shaped multimeric structure containing 100 glycans, among which, the changes in N-glycan during fibrosis can be identified using lectin WFA. M2BPGi is superior to existing biomarkers in distinguishing cases of advanced fibrosis (F3/F4; 3 COI or more indicates cirrhosis) and increased cirrhosis (up to 20 COI can be measured), and it markedly decreases following the successful treatment of HCV with interferon-based therapies (9,19).
Conversely, the CONUT score is a nutritional index that considers immune status and may be used as an index to evaluate various conditions, including the NST. To date, only a few studies have investigated the relationship between M2BPGi and ALBI grade or CONUT score in liver disease (20,21).
This is because the cut-off values for M2BPGi differ with the underlying cause of liver disease.
In this study, we examined M2BPGi cut-off values for each liver disease type. We found that M2BPGi was not a suitable biomarker for distinguishing between CONUT normal and light or low in outpatients; however, cut-offs by disease type were effective for other patients. Overall, M2BPGi presented the highest AUC in mALBI G1–G2a and G2b–G3 (AUC =0.88), and CONUT normal-light-moderate and severe (AUC =0.96) groups. Although distinguishing between CONUT normal and light-moderate is challenging, the finding that CONUT moderate and severe can be distinguished by a single marker suggests that active nutritional interventions can be expected at the outpatient group. In addition, the trend did not change significantly when the analysis was performed by primary disease. M2BPGi has also been reported to correlate with indices of the hepatic reserve, and it is speculated that Alb may contribute to this correlation. ALBI grade has also been reported to reflect nutritional status in patients with hepatocellular carcinoma (22).
Although these indices are important for the treatment of patients with hepatocellular carcinoma, shifts in M2BPGi have been reported in patients with hepatocellular carcinoma; thus, we excluded patients with hepatocellular carcinoma from this study. However, it is important to separate M2BPGi grade to decide the timing of treatment in clinical practice. The results of the present study demonstrated that M2BPGi can reflect ALBI grade and metabolic parameters, and thus, nutritional status as CONUT, although the cut-off varied with disease type.
This study had some limitations. First, data were obtained from a single institution. Second, the patients did not have a histological diagnosis of liver disease based on liver biopsy. Third, no indirect calorimetry assay was performed. Finally, all participants were outpatients and a few patients with Child C and ALBI 3 were included.
In the future, it will be necessary to validate these findings using data from a large number of patients obtained at multiple institutions, including hospitalized patients and patients with advanced CLD. In addition, it will be necessary to examine the use of this biomarker to predict carcinogenesis and to determine its relatedness to sarcopenia.
In conclusion, we found that M2BPGi is a single indicator of CLD status, including both ALBI grade and CONUT score.
Acknowledgments
We would like to thank Editage (https://www.editage.com) for English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-270/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-270/dss
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-270/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-270/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Institutional Review Board of Saiseikai Niigata Hospital (No. E18-18) and was conducted in accordance with the principles of the Declaration of Helsinki (as revised in 2013). All patients provided written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Johnson TM, Overgard EB, Cohen AE, et al. Nutrition assessment and management in advanced liver disease. Nutr Clin Pract 2013;28:15-29. [Crossref] [PubMed]
- Cheung K, Lee SS, Raman M. Prevalence and mechanisms of malnutrition in patients with advanced liver disease, and nutrition management strategies. Clin Gastroenterol Hepatol 2012;10:117-25. [Crossref] [PubMed]
- Tsiaousi ET, Hatzitolios AI, Trygonis SK, et al. Malnutrition in end stage liver disease: recommendations and nutritional support. J Gastroenterol Hepatol 2008;23:527-33. [Crossref] [PubMed]
- Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60:646-9. [Crossref] [PubMed]
- Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol 2015;33:550-8. [Crossref] [PubMed]
- Hiraoka A, Michitaka K, Kumada T, et al. Validation and Potential of Albumin-Bilirubin Grade and Prognostication in a Nationwide Survey of 46,681 Hepatocellular Carcinoma Patients in Japan: The Need for a More Detailed Evaluation of Hepatic Function. Liver Cancer 2017;6:325-36. [Crossref] [PubMed]
- Hiraoka A, Kumada T, Tsuji K, et al. Validation of Modified ALBI Grade for More Detailed Assessment of Hepatic Function in Hepatocellular Carcinoma Patients: A Multicenter Analysis. Liver Cancer 2019;8:121-9. [Crossref] [PubMed]
- Ignacio de Ulíbarri J, González-Madroño A, de Villar NG, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp 2005;20:38-45. [PubMed]
- Sasaki R, Yamasaki K, Abiru S, et al. Serum Wisteria Floribunda Agglutinin-Positive Mac-2 Binding Protein Values Predict the Development of Hepatocellular Carcinoma among Patients with Chronic Hepatitis C after Sustained Virological Response. PLoS One 2015;10:e0129053. [Crossref] [PubMed]
- Morio K, Imamura M, Daijo K, et al. Wisteria floribunda agglutinin positive Mac-2-binding protein level increases in patients with acute liver injury. J Gastroenterol 2017;52:1252-7. [Crossref] [PubMed]
- Ahn SS, Park Y, Lee DD, et al. Serum Wisteria floribunda agglutinin-positive Mac-2-binding protein can reflect systemic lupus erythematosus activity. Lupus 2018;27:771-9. [Crossref] [PubMed]
- Lai JC, Tandon P, Bernal W, et al. Malnutrition, Frailty, and Sarcopenia in Patients With Cirrhosis: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2021;74:1611-44. Erratum in: Hepatology 2021;74:3563. [Crossref] [PubMed]
- Tandon P, Raman M, Mourtzakis M, et al. A practical approach to nutritional screening and assessment in cirrhosis. Hepatology 2017;65:1044-57. [Crossref] [PubMed]
- Kuno A, Ikehara Y, Tanaka Y, et al. A serum "sweet-doughnut" protein facilitates fibrosis evaluation and therapy assessment in patients with viral hepatitis. Sci Rep 2013;3:1065. [Crossref] [PubMed]
- Ito K, Murotani K, Nakade Y, et al. Serum Wisteria floribunda agglutinin-positive Mac-2-binding protein levels and liver fibrosis: A meta-analysis. J Gastroenterol Hepatol 2017;32:1922-30. [Crossref] [PubMed]
- Kawaguchi K, Honda M, Ohta H, et al. Serum Wisteria floribunda agglutinin-positive Mac-2 binding protein predicts hepatocellular carcinoma incidence and recurrence in nucleos(t)ide analogue therapy for chronic hepatitis B. J Gastroenterol 2018;53:740-51. [Crossref] [PubMed]
- Miyaki E, Imamura M, Hiraga N, et al. Daclatasvir and asunaprevir treatment improves liver function parameters and reduces liver fibrosis markers in chronic hepatitis C patients. Hepatol Res 2016;46:758-64. [Crossref] [PubMed]
- Nagata H, Nakagawa M, Nishimura-Sakurai Y, et al. Serial measurement of Wisteria floribunda agglutinin positive Mac-2-binding protein is useful for predicting liver fibrosis and the development of hepatocellular carcinoma in chronic hepatitis C patients treated with IFN-based and IFN-free therapy. Hepatol Int 2016;10:956-64. [Crossref] [PubMed]
- Yamasaki K, Tateyama M, Abiru S, et al. Elevated serum levels of Wisteria floribunda agglutinin-positive human Mac-2 binding protein predict the development of hepatocellular carcinoma in hepatitis C patients. Hepatology 2014;60:1563-70. [Crossref] [PubMed]
- Eso Y, Takai A, Taura K, et al. Association of Mac-2-binding protein glycosylation isomer level with nutritional status in chronic liver disease. J Gastroenterol Hepatol 2018; Epub ahead of print. [Crossref] [PubMed]
- Nishikawa H, Enomoto H, Yoh K, et al. Combined albumin-bilirubin grade and Mac-2 binding protein glycosylation isomer as a useful predictor in compensated liver cirrhosis. Medicine (Baltimore) 2019;98:e18366. [Crossref] [PubMed]
- Kotoh Y, Saeki I, Yamasaki T, et al. Albumin-bilirubin score as a useful predictor of energy malnutrition in patients with hepatocellular carcinoma. Clin Nutr 2021;40:3585-91. [Crossref] [PubMed]


