
Longitudinal symptoms and temporal trends in palliative care, palliative radiotherapy, and anti-cancer treatment near end of life among patients with metastatic cancer
Introduction
Several randomized controlled trials have demonstrated the benefits of concurrent specialty palliative care (PC) with oncologic care, with enhanced patient outcomes such as better quality of life and mood (1-5). Early supportive care is also associated with improved clinical symptoms and less intensive end-of-life interventions (6,7).
Both specialty PC and palliative radiotherapy (RT) are integral pillars of symptom-directed treatment for patients with metastatic disease. Specialty PC may be helpful for reducing pain and psychological distress, providing skills to cope effectively with life-threatening illness, and facilitating improved communication alongside understanding of goals and prognosis (8-11). Similarly, palliative RT may be prescribed to ameliorate pain and other symptoms, reduce the risk of impending pathologic fracture, or improve local control to minimize further symptom progression or functional compromise (12-16). Understanding longitudinal and temporal patterns in the delivery of specialty PC and palliative RT—which often aim to achieve complementary, but distinct, goals—is important for optimal coordination of multidisciplinary oncologic care.
At the time of diagnosis of incurable cancer, many patients experience a high symptom burden including pain and fatigue (17-19). Nearly 80% of patients with metastatic cancer also present with symptomatic disease at the time of palliative RT consultation (20). Many patients with advanced cancer experience emotional burdens in addition to physical symptoms related to their disease and treatment side effects (21). Existing literature lack granular data on patterns of physical and psychosocial symptoms across palliative RT and specialty PC visits among patients with advanced cancer. Hence, this study assesses longitudinal trends in specialty PC, palliative RT, and symptom distribution across the survival continuum of metastatic cancer. Additionally, this study explores clinical and symptom-based predictors of earlier specialty PC utilization. We present the following article in accordance with the STROBE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-301/rc).
Methods
Study population
We performed a retrospective review of patients with metastatic, non-hematologic, solid malignancies who received palliative RT at our institution from July 2017 to February 2018 and who had died by the date of final study follow-up in June 2021 as ascertained by the electronic medical records. A larger cohort of 162 patients with metastatic cancer was initially identified and has been previously described (20). From the earlier cohort, a subset of 135 patients who had died and were followed for a longer duration is described in the current study. Patients were included if they had a diagnosis of metastatic, incurable, solid malignancy, were age ≥18 years at the time of first-course palliative RT, and had died by the date of last follow-up. Patients with hematologic malignancies were not included in this study as they are not commonly treated with palliative RT and often have a disease trajectory distinct from those with metastatic solid malignancies. Palliative RT was defined as RT delivered with non-curative intent to a metastatic site or primary site for symptom control. Patients were excluded from the study if they received prior courses of palliative RT, did not receive palliative RT, and had international home addresses to minimize loss to follow-up and potential bias. Patients were referred to specialty PC by their treating physicians, including their inpatient medical teams, medical oncologists, radiation oncologists, or via self-referral. There were no specific referral criteria other than the treating physicians’ clinical judgment of the need for specialty PC. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Dana-Farber Cancer Institute/Brigham and Women’s Cancer Center Institutional Review Board (protocol No. 17-669) and individual consent for the retrospective analysis was waived.
Study parameters and endpoints
We reviewed the electronic medical records for patients’ demographic and clinical characteristics, which included age, sex, patient-reported race/ethnicity, language, performance status, tumor histology, burden of metastatic disease, number of prior palliative chemotherapy regimens, RT treatment courses, hospital admissions, hospice referrals, and symptoms at all specialty PC visits, as documented by the treating specialty PC provider.
Relative survival time was determined based on quartiles of life remaining from metastatic diagnosis to death. Quartiles 1–3 represent the first 75% of life remaining from the date of metastatic diagnosis to date of death, whereas quartile 4 represents the last 25% of life remaining. We defined earlier specialty PC as occurring in quartiles 1–3 and later specialty PC as occurring in quartile 4. Previous studies support time-based criteria for early integration of specialty PC, including within 2–3 months of diagnosis of advanced or incurable cancer (3,22,23). The primary outcomes included distributions of specialty PC, palliative RT, admissions, hospice referral, and symptoms over relative survival time. The secondary outcomes included predictors of earlier specialty PC utilization and incidence of hospice referral based on timing of first specialty PC visit.
Statistical analysis
We used descriptive statistics to summarize frequencies of clinical visits and symptoms experienced over relative survival time, comparing the first 75% (quartiles 1–3) and last 25% (quartile 4) of life remaining from metastatic diagnosis to death. Interquartile ranges (IQR) were used to describe the spread of non-normally distributed data. We used univariable and multivariable logistic regression analyses to determine predictors of receiving earlier (quartiles 1–3) versus later (quartile 4) specialty PC. We used Fisher’s exact test to examine differences in single versus multi-fraction palliative RT, hospice referral incidence, and anti-cancer treatment or palliative RT within 30 days of death for earlier versus later specialty PC. Patients with unknown demographic characteristics were excluded from logistic regression analyses. Odds ratios (OR) were reported with a 95% confidence interval (CI). The statistical significance was defined as a two-sided P value of less than 0.05. Analyses were conducted using R version 3.6.3.
Results
Patient characteristics
The median age of patients at the time of diagnosis of metastatic cancer was 62 years (IQR, 53–69) and the median age of patients at the time of palliative RT was 63 years (IQR, 56–72). Among the 135 patients in the cohort, most patients were female (72/135, 53.3%), white (117/135, 86.7%), and English-speaking (115/135, 85.2%) (Table 1). Forty-seven patients (34.8%) had primary lung histology, 37 patients (27.4%) had primary breast or prostate histology, and 51 patients (37.8%) had other primary histology. There were 7 patients with unknown preferred language, 3 patients with unknown race, and 2 patients with unknown marital status. Patients were treated with a median RT dose of 2,500 cGy (IQR, 2,000–3,000) in a median of 5 fractions (IQR, 5–10).
Table 1
| Variable | n | % |
|---|---|---|
| Age at palliative RT | ||
| <65 years | 71 | 52.6 |
| ≥65 years | 64 | 47.4 |
| Sex | ||
| Female | 72 | 53.3 |
| Male | 63 | 46.7 |
| Race | ||
| White | 117 | 86.7 |
| Asian | 7 | 5.2 |
| Black | 5 | 3.7 |
| Other | 3 | 2.2 |
| Unknown | 3 | 2.2 |
| Marital status | ||
| Married or life partner | 96 | 71.1 |
| Single | 20 | 14.8 |
| Widowed or divorced | 17 | 12.6 |
| Unknown | 2 | 1.5 |
| Language | ||
| English | 115 | 85.2 |
| Non-Englisha | 13 | 9.6 |
| Unknown | 7 | 5.2 |
| Travel distance | ||
| ≤25 miles | 55 | 40.7 |
| 26–50 miles | 46 | 34.1 |
| >50 miles | 34 | 25.2 |
| Primary tumor histology | ||
| Breast/prostate | 37 | 27.4 |
| Lung | 47 | 34.8 |
| Otherb | 51 | 37.8 |
| Metastatic burdenc | ||
| 1 | 25 | 18.5 |
| 2 | 40 | 29.6 |
| 3 | 43 | 31.9 |
| ≥4 | 27 | 20.0 |
| Number of lines of prior palliative chemotherapy | ||
| 0 | 17 | 12.6 |
| 1 | 40 | 29.6 |
| 2 | 23 | 17.0 |
| 3 | 27 | 20.0 |
| ≥4 | 28 | 20.7 |
| ECOG performance status | ||
| 0 | 33 | 24.4 |
| 1 | 52 | 38.5 |
| 2 | 31 | 23.0 |
| ≥3 | 19 | 14.1 |
a, non-English languages spoken by patients include Spanish, Cantonese, French, Persian, Haitian-Creole, Japanese, Russian, Arabic, and American Sign Language; b, other histology include stomach, mesothelioma, neuroendocrine, esophageal, renal, genitourinary, gynecologic, colorectal, head and neck, hepatocellular, melanoma, cholangiocarcinoma, pancreas, sarcoma, small bowel, and thyroid; c, metastatic burden refers to the number of systems with metastatic disease (e.g., lung, liver, bone, etc.). Multiple sites of involvement for 1 system (e.g., 3 osseous metastases) were counted as having a metastatic burden of 1. RT, radiotherapy; ECOG, Eastern Cooperative Oncology Group.
Distributions of clinical visits over relative survival time
At the time of diagnosis of metastatic cancer, the median overall survival (OS) among all patients was 33.5 months, with the last 25% of life representing a median of 8.4 months of survival remaining. The overall cohort represented 436.5 person-years of follow-up time. There was no appreciable difference in months of median OS among patients who received specialty PC (33.5 months) versus those who did not receive specialty PC (33.4 months).
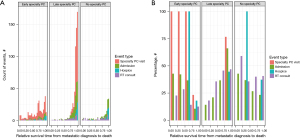
There were 16.3% (22/135), 10.4% (14/135), 26.7% (36/135), and 46.7% (63/135) of all palliative RT consultations occurring in quartiles 1, 2, 3, and 4, respectively. In contrast, 4.7% (27/577), 7.6% (44/577), 14.0% (81/577), and 73.7% (425/577) of all specialty PC visits occurred in quartiles 1, 2, 3, and 4, respectively. Compared to the number of occurrences in quartiles 1–3, more hospital admissions (236/292, 80.8%) occurred in quartile 4, and nearly all hospice admissions (94/99, 95.0%) occurred in quartile 4. Figure 1 depicts the overall timing and frequencies of palliative RT consultations, specialty PC visits, admissions, and hospice referrals over relative survival time, while Figure 2 illustrates each event stratified by receipt of earlier specialty PC compared to later or no specialty PC in absolute counts (Figure 2A) and percentages (Figure 2B). In the last quartile of life remaining from metastatic diagnosis to death, 76.7% of specialty PC visits occurred among patients who received later specialty PC compared to 23.3% among patients who received earlier specialty PC. A greater percentage of patients (46.9% versus 12.5%) received palliative RT in the last quartile among those who received later versus earlier specialty PC, respectively. There was no difference in use of single versus multi-fraction RT among patients who received earlier, later, or no specialty PC (P=0.58).
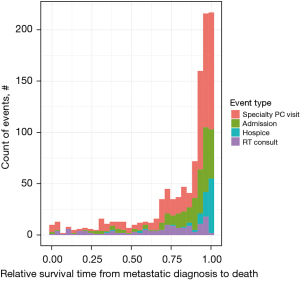
Distributions of symptoms over relative survival time
Among the 135 study patients, 77 received specialty PC for a total of 577 specialty PC visits. The median number of specialty PC visits per patient was 5 (IQR, 2–8). The first specialty PC visit occurred in the inpatient setting for 48.1% of patients (37/77) and outpatient setting for 51.9% of patients (40/77). No palliative home visits occurred in this study population. Most patients were referred to specialty PC by an inpatient care team (37/77, 48.1%) or their medical oncologist (36/77, 46.8%). Only one patient each was referred by a radiation oncologist (1/77, 1.3%) or self-referred to specialty PC (1/77, 1.3%). The referral team for two patients (2/77, 2.6%) was unknown. The most common primary symptoms addressed or topic of conversation at the first specialty PC visit was pain (47/77, 61.0%), followed by goals of care (15/77, 19.5%), psychosocial support (6/77, 7.8%), physical symptoms other than pain (5/77, 6.5%), and end-of-life symptom management (4/77, 5.2%). There were 28 patients (36.4%) for whom addressing physical symptoms other than pain was a secondary reason for specialty PC consultation.
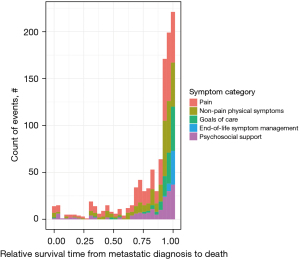
Across the relative survival continuum, pain was addressed proportionally more frequently in quartiles 1–3 (131/254, 51.6%) compared to quartile 4 (283/795, 35.6%) (Table 2, Figure 3). Goals of care and end-of-life symptom management were addressed more frequently in quartile 4 (172/795, 21.6%) compared to quartiles 1–3 (7/254, 2.8%). Non-pain physical symptoms were addressed proportionally more evenly across quartiles 1–3 (73/254, 28.7%) and quartile 4 (219/795, 27.5%).
Table 2
| Quartile | Pain (n=414) | Non-pain physical symptom (n=292) | Goals of care (n=127) | End-of-life symptom management (n=45) | Psychosocial support (n=171) | Total number of symptoms in each quartile |
|---|---|---|---|---|---|---|
| Quartile 1 | 25 (6.0%) | 12 (4.1%) | 1 (0.8%) | 0 | 14 (8.2%) | 52 |
| Quartile 2 | 36 (8.7%) | 25 (8.6%) | 3 (2.4%) | 0 | 8 (4.7%) | 72 |
| Quartile 3 | 70 (16.9%) | 36 (12.3%) | 3 (2.4%) | 0 | 21 (12.3%) | 130 |
| Quartile 4 | 283 (68.4%) | 219 (75.0%) | 120 (94.5%) | 45 (100%) | 128 (74.9%) | 795 |
a, the median duration for each quartile is 8.4 months. PC, palliative care.
Predictors of earlier specialty PC
On univariable analysis, pain as a presenting symptom at the first specialty PC visit was associated with earlier utilization of specialty PC (OR =17.15; 95% CI: 3.20–318.95; P=0.007) (Table 3). In contrast, goals of care conversations during the first specialty PC visit (OR =0.25; 95% CI: 0.05–0.84; P=0.041), receiving ≥2 prior lines of palliative chemotherapy (OR =0.33; 95% CI: 0.11–0.94; P=0.040), and referral to specialty PC by an inpatient care team compared to other referral sources (OR =0.21; 95% CI: 0.06–0.63; P=0.008) were associated with decreased likelihood of receiving earlier specialty PC.
Table 3
| Variable | Univariable analyses | Multivariable analyses | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Age | |||||
| <65 years | Reference | ||||
| ≥65 years | 0.49 (0.17–1.36) | 0.175 | – | – | |
| Sex | |||||
| Female | Reference | ||||
| Male | 2.73 (0.97–8.27) | 0.064 | – | – | |
| Language | |||||
| English | Reference | ||||
| Non-English | 0.24 (0.01–1.40) | 0.188 | – | – | |
| Travel distance | |||||
| ≤25 miles | Reference | ||||
| ≥26 miles | 1.85 (0.66–5.58) | 0.254 | – | – | |
| Primary tumor histology | |||||
| Breast/prostate | Reference | ||||
| Lung | 1.67 (0.37–8.05) | 0.508 | – | – | |
| Other | 2.00 (0.58–8.16) | 0.295 | – | – | |
| Metastatic burdenb | |||||
| 1 | Reference | ||||
| ≥2 | 1.01 (0.29–4.09) | 0.986 | – | – | |
| Number of lines of prior palliative chemotherapy | |||||
| ≤1 | Reference | Reference | |||
| ≥2 | 0.33 (0.11–0.94) | 0.040 | 0.16 (0.04–0.58) | 0.009 | |
| ECOG performance status | |||||
| ≤1 | Reference | ||||
| ≥2 | 1.42 (0.49–4.00) | 0.510 | – | – | |
| Referral team | |||||
| Medical oncologist/radiation oncologist/self-referral | Reference | Reference | |||
| Inpatient care team | 0.21 (0.06–0.63) | 0.008 | 0.26 (0.05–1.06) | 0.074 | |
| Symptom/topic addressed at first specialty PC visitc | |||||
| Pain | 17.15 (3.20–318.95) | 0.007 | 15.34 (2.16–324.23) | 0.020 | |
| Physical symptoms other than pain | 0.98 (0.35–2.72) | 0.972 | – | – | |
| Goals of care | 0.25 (0.05–0.84) | 0.041 | 0.77 (0.13–3.91) | 0.755 | |
| Psychosocial support | 1.33 (0.40–4.12) | 0.623 | – | – | |
a, four patients with unknown primary language were excluded from logistic regression analyses; b, metastatic burden refers to the number of systems with metastatic disease (e.g., lung, liver, bone, etc.). Multiple sites of involvement for 1 system (e.g., 3 osseous metastases) were counted as having a metastatic burden of 1; c, multiple symptoms were possible for each patient. Reference level is the absence of the symptom. End-of-life symptom management was not included due to a small number of patients who presented with this concern at the first specialty PC visit. PC, palliative care; OR, odds ratios; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group.
On multivariable analysis, pain as a presenting symptom for specialty PC was an independent predictor of receiving earlier PC (OR =15.34; 95% CI: 2.16–324.23; P=0.020), while patients who received ≥2 prior lines of chemotherapy were less likely to have received earlier specialty PC (OR =0.16; 95% CI: 0.04–0.58; P=0.009). There was a trend towards patients referred by their inpatient care team being less likely to have received earlier specialty PC (OR =0.26; 95% CI: 0.05–1.06; P=0.074).
Hospice referral outcomes
There were 99 patients (99/135, 73.3%) who were referred to hospice, including home with hospice services, inpatient hospice, or a non-hospital affiliated hospice facility. Of the 77 patients who received specialty PC, 62 (80.5%) were referred to hospice after a median of 62.5 days following their first specialty PC visit (IQR, 24.5–187 days). There was no difference between frequency of hospice referral for those receiving their first specialty PC visit in relative survival quartiles 1–3 versus quartile 4 (OR =3.06; 95% CI: 0.60–30.49; P=0.21).
Of the 36 patients who were not referred to hospice, 16 (44.4%) died before hospice could be set up, 13 (36.1%) were still receiving anti-cancer treatment, 3 (8.3%) declined hospice, and 4 (11.1%) did not have an identifiable reason for hospice non-referral. Overall, thirteen patients (13/135, 9.6%) received either chemotherapy (n=6), radiation therapy (n=6), or surgery (n=1) within the last 30 days of death. Among patients who received versus did not receive earlier specialty PC, there were no differences in the incidence of anti-cancer treatment (P=0.44) or palliative RT (P=1.00) within the last 30 days of death.
Discussion
Improved care coordination and communication between specialized PC and oncologic teams, including radiation oncologists, may lead to better quality of life and clinical outcomes for patients with advanced cancers (24,25). Towards this goal, our study is the first to our knowledge to assess longitudinal patterns in the delivery of palliative RT and specialty PC, in addition to the distribution of symptoms over relative survival time from date of metastatic diagnosis among patients receiving palliative RT. We found that most specialty PC visits but not palliative RT consultations occurred in the last quarter of life from metastatic diagnosis to death and that several patients received anti-cancer treatment in the 30 days preceding death. We also found that both presence of pain and fewer lines of prior chemotherapies were predictors for earlier specialty PC. A key implication of these data is that initiating referrals to specialty PC at the time of palliative RT may facilitate evaluation for and co-management of patients’ symptoms in a goal-concordant fashion.
In our study, intensification of the frequency of specialty PC visits over relative survival time likely reflects acknowledgement by oncologic care teams that specialty PC offers valuable support for patients in a vulnerable end-of-life period. However, the lack of earlier initiation of specialty PC in our cohort may indicate a pervasive misunderstanding that PC is synonymous with end-of-life care, which may induce avoidance patterns and make early disclosure of poor prognosis more difficult for oncologists (26). Despite advancements in our understanding of the beneficial impacts of specialty PC in the past decade, barriers to PC initiation remain, including provider-level barriers such as lack of PC knowledge and reluctance to initiate referrals, and patient-level barriers, such as financial challenges, cultural differences, and negative perceptions of PC among patients and their caregivers (27-29). In contrast to most specialty PC referrals occurring in the last 25% of life remaining, palliative RT was more evenly distributed along the survival continuum. This comparatively earlier occurrence of palliative RT consultations presents a unique opportunity for radiation oncologists to not only assess patients’ symptoms for the purpose of determining utility of palliative RT, but also to co-manage symptoms and refer patients (either directly or in collaboration with the medical oncologist) to specialty PC. Integrating the perspectives of radiation oncologists as they’re seeing patients may provide an opportunity for more streamlined and timelier referral of patients to specialty PC. Compared to one-time interventions or specialty PC consultations offered on demand, early and systematic integration of specialty PC is more beneficial for patients with advanced cancer (3,30). Compellingly, we found that earlier initiation of specialty PC was associated with decreased hospital admissions and specialty PC utilization near the end of life. As a patient’s PC needs can vary throughout their disease course, longitudinal delivery of specialty PC is needed to sustain the benefits of early PC (31). In radiation oncology, while approximately half of patients who receive RT are treated with palliative intent, dedicated PC training is often lacking in radiation oncology training programs. PC training for radiation oncologists is an important precursor to increasing radiation oncologists’ participation in the multidisciplinary PC process (32,33). Initiatives to educate and empower radiation oncologists to refer patients to specialty PC as part of standard workflows may facilitate shifting specialty PC more upstream for patients with advanced cancer.
Relatedly, acute cancer-associated pain is often underassessed at the time of radiation oncology consultation (33). As such, patients with pain related to metastatic disease, who are often referred for palliative RT, represent a group who may particularly benefit from process improvements which lead to earlier integration of specialty PC with oncologic care. In accordance with a previous study showing that referral for pain and symptom management was associated with earlier referral to specialty PC (34), we found that the presence of pain predicted for earlier specialty PC integration. Interestingly, physical symptoms other than pain were addressed in similar proportions across each quartile of survival time since metastatic diagnosis, whereas pain was proportionally more predominant in the first three quartiles and conversations related to goals of care were more predominant in the last quartile of remaining life. One explanation for this trend is that physical symptoms other than pain may be more difficult to alleviate with current specialty PC tools and are revisited across time as new physical symptoms develop or worsen with disease progression. These findings underscore the need for both early symptom screening and adequate PC support and services throughout the disease trajectory to address patients’ symptoms. Given that patient-reported distress is an early warning sign of unmet PC needs and is associated with increased utilization of emergency room and inpatient services (35), an improved characterization of patients’ symptoms may empower clinicians to break spiraling cycles of distress.
Among patients who received palliative chemotherapy, patient- and provider-level preferences, such as holding out for more aggressive therapy, may have contributed to a delay in referral to specialty PC for patients who received multiple prior palliative chemotherapy regimens. This is related to our finding that goals of care conversations were concentrated primarily in the last 25% of life remaining. A recent study has shown that opportunities for conversations on end of life may often be missed, with oncologists responding inadequately to patient concerns over disease progression or dying by using optimistic future talk to address patient concerns or expressing concern over treatment discontinuation (36). Physicians may also be concerned that mentioning PC may increase patients’ distress or diminish their sense of hope (37,38). In addition, patients with advanced cancer frequently hold inaccurate perceptions of treatment goals and prognosis, with many patients receiving palliative chemotherapy believing that the intent of chemotherapy is curative, despite receiving educational materials that suggest otherwise (39,40). Furthermore, patients who overestimate their chance of survival are more likely to receive aggressive care at the end of life (41). On the other hand, early PC facilitates empathic communication that may sustain patients’ hope and psychological well-being (42,43). Facilitating earlier discussions of goals and prognosis may increase receptiveness to specialty PC, decrease aggressiveness in end-of-life anti-cancer treatment and healthcare utilizations, and improve quality of life outcomes near the end of life.
Finally, although all patients in our study cohort had died, nearly 10% of patients received anti-cancer treatment within 30 days preceding death and 73.3% of patients were referred to hospice. While we did not find a statistically significant difference between incidence of hospice referral for those receiving earlier versus later specialty PC, another retrospective study of advanced cancer patients found that 3.4% and 24.6% of patients who received early versus delayed PC, respectively, had received chemotherapy in the last 60 days of life (44). Among a large retrospective cohort of patients who received palliative RT, 24% of patients had received palliative RT within 30 days of death (45). Prior studies have shown that clinicians are often not accurate at predicting prognosis and life expectancies for patients (46,47), which may have contributed to anti-cancer treatment near end of life. The development of clinical tools and models to assist in more accurate prognostication may facilitate appropriate earlier referrals to specialty PC. Initiatives to improve clinicians’ communication skills may also lead to increased patient and caregiver comfort regarding transitions in goals of care from anti-cancer treatment to hospice and may result in earlier specialty PC intervention for patients with metastatic cancer.
This study is limited in the retrospective nature of data collection from patient medical records; we are only able to examine the trends in symptoms that are reported by patients and subsequently documented by their treating physicians. It is possible that symptoms may not have been elicited from patients or symptoms may have been reported and not documented. In addition, this study focused on a cohort of patients with metastatic cancer and does not include patients with incurable advanced localized disease without metastases who may receive palliative RT. The sample size is relatively small at 135 patients, although this sample size allowed us to capture longitudinal data, which was the intended goal of this study, across over 1,100 different visits and 400 person-years. The patients in this cohort were relatively homogenous, with few patients who were non-white or whose primary languages were not English. The patient population was also relatively young, which may reflect community referral patterns or preferences of patients in seeking care at a tertiary academic institution, which may present limitations to generalizability. Additional research is needed to determine how these findings may differ in larger or more diverse patient populations.
Conclusions
Nearly 74% of specialty PC visits occurred in the last quarter of life from metastatic diagnosis to death, compared to approximately 47% of palliative RT visits occurring in the same time frame. Patients who reported experiencing pain and those who received one or fewer prior palliative chemotherapy regimens were more likely to have received earlier specialty PC. Although all patients in this study had died, 73% of patients were referred to hospice and nearly 10% of patients had received chemotherapy, radiation therapy, or surgery within 30 days preceding death, which reflect opportunities for improving clinician recognition of end of life, as well as communication with patients and their families about goals of care. Along with identifying factors and symptom triggers to prompt addressing patients’ PC needs sooner, coordinated multidisciplinary care to offer goal-concordant care is needed, which may decrease aggressiveness in end-of-life anti-cancer treatment. Referrals to specialty PC at the time of palliative RT may be an intervention that facilitates earlier involvement of specialty PC in the disease trajectory for patients with advanced cancer.
Acknowledgments
Aspects of this work were presented at the 63rd Annual Meeting of the American Society for Radiation Oncology in Chicago, IL, October 24–27, 2021.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-301/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-301/dss
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-301/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-301/coif). TAB serves as an unpaid editorial board member of Annals of Palliative Medicine from April 2014 to January 2024. In the past 36 months, MAH has been supported by grants from ViewRay and the Dana Farber Cancer Institute. These grants are not related to the present manuscript. AS reports receiving grants from the Burroughs-Wellcome Fund, National Cancer Institute, and Dana-Farber Cancer Institute for work that are not related to the present manuscript. In the past 36 months, TAB has been supported by grants from the National Institutes of Health and the Templeton Foundation. These grants are not related to the present manuscript. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Dana-Farber Cancer Institute/Brigham and Women’s Cancer Center Institutional Review Board (protocol No. 17-669) and individual consent for the retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010;363:733-42. [Crossref] [PubMed]
- Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet 2014;383:1721-30. [Crossref] [PubMed]
- Vanbutsele G, Pardon K, Van Belle S, et al. Effect of early and systematic integration of palliative care in patients with advanced cancer: a andomized controlled trial. Lancet Oncol 2018;19:394-404. [Crossref] [PubMed]
- Bakitas MA, Tosteson TD, Li Z, et al. Early Versus Delayed Initiation of Concurrent Palliative Oncology Care: Patient Outcomes in the ENABLE III Randomized Controlled Trial. J Clin Oncol 2015;33:1438-45. [Crossref] [PubMed]
- Nottelmann L, Groenvold M, Vejlgaard TB, et al. Early, integrated palliative rehabilitation improves quality of life of patients with newly diagnosed advanced cancer: The Pal-Rehab randomized controlled trial. Palliat Med 2021;35:1344-55. [Crossref] [PubMed]
- Chan KY, Gill H, Chan TSY, et al. Early integrated palliative care for haematology cancer patients-the impact on symptom burden in Hong Kong. Ann Palliat Med 2021;10:6316-24. [Crossref] [PubMed]
- Potenza L, Scaravaglio M, Fortuna D, et al. Early palliative/supportive care in acute myeloid leukaemia allows low aggression end-of-life interventions: observational outpatient study. BMJ Support Palliat Care 2021; Epub ahead of print. [Crossref] [PubMed]
- Bickel KE, McNiff K, Buss MK, et al. Defining High-Quality Palliative Care in Oncology Practice: An American Society of Clinical Oncology/American Academy of Hospice and Palliative Medicine Guidance Statement. J Oncol Pract 2016;12:e828-38. [Crossref] [PubMed]
- Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA 2009;302:741-9. [Crossref] [PubMed]
- Greer JA, Jacobs JM, El-Jawahri A, et al. Role of Patient Coping Strategies in Understanding the Effects of Early Palliative Care on Quality of Life and Mood. J Clin Oncol 2018;36:53-60. [Crossref] [PubMed]
- Zimmermann C, Riechelmann R, Krzyzanowska M, et al. Effectiveness of specialized palliative care: a systematic review. JAMA 2008;299:1698-709. [Crossref] [PubMed]
- Williams GR, Manjunath SH, Butala AA, et al. Palliative Radiotherapy for Advanced Cancers: Indications and Outcomes. Surg Oncol Clin N Am 2021;30:563-80. [Crossref] [PubMed]
- Chow E, Harris K, Fan G, et al. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol 2007;25:1423-36. [Crossref] [PubMed]
- Chow E, van der Linden YM, Roos D, et al. Single versus multiple fractions of repeat radiation for painful bone metastases: a andomized, controlled, non-inferiority trial. Lancet Oncol 2014;15:164-71. [Crossref] [PubMed]
- Lutz S, Balboni T, Jones J, et al. Palliative radiation therapy for bone metastases: Update of an ASTRO Evidence-Based Guideline. Pract Radiat Oncol 2017;7:4-12. [Crossref] [PubMed]
- Chen JJ, Sullivan AJ, Shi DD, et al. Characteristics and Predictors of Radiographic Local Failure in Patients With Spinal Metastases Treated With Palliative Conventional Radiation Therapy. Adv Radiat Oncol 2021;6:100665. [Crossref] [PubMed]
- Teunissen SC, Wesker W, Kruitwagen C, et al. Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manage 2007;34:94-104. [Crossref] [PubMed]
- Vogt J, Beyer F, Sistermanns J, et al. Symptom Burden and Palliative Care Needs of Patients with Incurable Cancer at Diagnosis and During the Disease Course. Oncologist 2021;26:e1058-65. [Crossref] [PubMed]
- Luo J, Chen JJ, Deguzman C, et al. Symptoms and palliative care needs of pancreatic adenocarcinoma patients. J Palliat Med 2014;17:640-1. [Crossref] [PubMed]
- Chen JJ, Rawal B, Krishnan MS, et al. Patterns of Specialty Palliative Care Utilization Among Patients Receiving Palliative Radiation Therapy. J Pain Symptom Manage 2021;62:242-51. [Crossref] [PubMed]
- Kebebew T, Mavhandu-Mudzusi AH, Mosalo A. A cross-sectional assessment of symptom burden among patients with advanced cervical cancer. BMC Palliat Care 2021;20:190. [Crossref] [PubMed]
- Hui D, Anderson L, Tang M, et al. Examination of referral criteria for outpatient palliative care among patients with advanced cancer. Support Care Cancer 2020;28:295-301. [Crossref] [PubMed]
- Hui D, Heung Y, Bruera E. Timely Palliative Care: Personalizing the Process of Referral. Cancers (Basel) 2022;14:1047. [Crossref] [PubMed]
- Dans M, Kutner JS, Agarwal R, et al. NCCN Guidelines® Insights: Palliative Care, Version 2.2021. J Natl Compr Canc Netw 2021;19:780-8. [Crossref] [PubMed]
- Smith TJ, Temin S, Alesi ER, et al. American Society of Clinical Oncology provisional clinical opinion: the integration of palliative care into standard oncology care. J Clin Oncol 2012;30:880-7. [Crossref] [PubMed]
- Sarradon-Eck A, Besle S, Troian J, et al. Understanding the Barriers to Introducing Early Palliative Care for Patients with Advanced Cancer: A Qualitative Study. J Palliat Med 2019;22:508-16. [Crossref] [PubMed]
- Enguidanos S, Cardenas V, Wenceslao M, et al. Health Care Provider Barriers to Patient Referral to Palliative Care. Am J Hosp Palliat Care 2021;38:1112-9. [Crossref] [PubMed]
- Mayeda DP, Ward KT. Methods for overcoming barriers in palliative care for ethnic/racial minorities: a systematic review. Palliat Support Care 2019;17:697-706. [Crossref] [PubMed]
- Zimmermann C, Swami N, Krzyzanowska M, et al. Perceptions of palliative care among patients with advanced cancer and their caregivers. CMAJ 2016;188:E217-27. [Crossref] [PubMed]
- Eychmüller S, Zwahlen S, Fliedner MC, et al. Single early palliative care intervention added to usual oncology care for patients with advanced cancer: A randomized controlled trial (SENS Trial). Palliat Med 2021;35:1108-17. [Crossref] [PubMed]
- Murray SA, Kendall M, Mitchell G, et al. Palliative care from diagnosis to death. BMJ 2017;356:j878. [Crossref] [PubMed]
- Tseng YD, Krishnan MS, Jones JA, et al. Supportive and palliative radiation oncology service: impact of a dedicated service on palliative cancer care. Pract Radiat Oncol 2014;4:247-53. [Crossref] [PubMed]
- Garcia MA, Braunstein SE, Anderson WG. Palliative Care Didactic Course for Radiation Oncology Residents. Int J Radiat Oncol Biol Phys 2017;97:884-5. [Crossref] [PubMed]
- Wadhwa D, Popovic G, Pope A, et al. Factors Associated with Early Referral to Palliative Care in Outpatients with Advanced Cancer. J Palliat Med 2018;21:1322-8. [Crossref] [PubMed]
- Hildenbrand JD, Park HS, Casarett DJ, et al. Patient-reported distress as an early warning sign of unmet palliative care needs and increased healthcare utilization in patients with advanced cancer. Support Care Cancer 2022;30:3419-27. [Crossref] [PubMed]
- Knutzen KE, Sacks OA, Brody-Bizar OC, et al. Actual and Missed Opportunities for End-of-Life Care Discussions With Oncology Patients: A Qualitative Study. JAMA Netw Open 2021;4:e2113193. [Crossref] [PubMed]
- Quill TE. Perspectives on care at the close of life. Initiating end-of-life discussions with seriously ill patients: addressing the “elephant in the room”. JAMA 2000;284:2502-7. [Crossref] [PubMed]
- Broom A, Kirby E, Good P, et al. The troubles of telling: managing communication about the end of life. Qual Health Res 2014;24:151-62. [Crossref] [PubMed]
- Weeks JC, Catalano PJ, Cronin A, et al. Patients’ expectations about effects of chemotherapy for advanced cancer. N Engl J Med 2012;367:1616-25. [Crossref] [PubMed]
- Enzinger AC, Uno H, McCleary N, et al. Effectiveness of a Multimedia Educational Intervention to Improve Understanding of the Risks and Benefits of Palliative Chemotherapy in Patients With Advanced Cancer: A Randomized Clinical Trial. JAMA Oncol 2020;6:1265-70. [Crossref] [PubMed]
- Weeks JC, Cook EF, O’Day SJ, et al. Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA 1998;279:1709-14. Erratum in: JAMA 2000;283:203. [Crossref] [PubMed]
- Kaasa S, Loge JH, Aapro M, et al. Integration of oncology and palliative care: a Lancet Oncology Commission. Lancet Oncol 2018;19:e588-653. [Crossref] [PubMed]
- Hagerty RG, Butow PN, Ellis PM, et al. Communicating with realism and hope: incurable cancer patients’ views on the disclosure of prognosis. J Clin Oncol 2005;23:1278-88. Erratum in: J Clin Oncol 2005;23:3652. [Crossref] [PubMed]
- Bandieri E, Banchelli F, Artioli F, et al. Early versus delayed palliative/supportive care in advanced cancer: an observational study. BMJ Support Palliat Care 2020;10:e32. [Crossref] [PubMed]
- Wu SY, Singer L, Boreta L, et al. Palliative radiotherapy near the end of life. BMC Palliat Care 2019;18:29. [Crossref] [PubMed]
- Gensheimer MF, Aggarwal S, Benson KRK, et al. Automated model versus treating physician for predicting survival time of patients with metastatic cancer. J Am Med Inform Assoc 2021;28:1108-16. [Crossref] [PubMed]
- Benson KRK, Aggarwal S, Carter JN, et al. Predicting Survival for Patients With Metastatic Disease. Int J Radiat Oncol Biol Phys 2020;106:52-60. [Crossref] [PubMed]


