
The safety and urate-lowering efficacy of febuxostat in patients undergoing peritoneal dialysis: a retrospective single-arm cohort study of 84 patients
Introduction
Uric acid (UA) is the final enzymatic product of purine or nucleotide metabolism produced by xanthine oxidase or xanthine dehydrogenase, approximately two-thirds of which is excreted through the kidneys and the remainder is excreted via the gastrointestinal tracts (1,2). Gout is an inflammatory type of arthritis which is commonly thought to be caused by deposition of monosodium urate crystals, secondary to increased serum UA (sUA) concentration (3). Hyperuricemia (HUA) is often encountered in patients with chronic kidney disease (CKD) and end-stage renal disease (ESRD) owing to declining kidney function. Not only has HUA been demonstrated to result in gout, but it is also associated with CKD, hypertension, cardiovascular disease (CVD), and diabetes (4-8). Accumulating epidemiological studies have confirmed the association between higher sUA level and CVD as well as mortality in CKD and ESRD patients, indicating the importance of urate-lowering therapy in these patients (9-11).
Febuxostat, a new type of non-purine selective xanthine oxidase inhibitor, is mainly metabolized in the liver and is excreted through the biliary tract and kidneys (12). It is excreted in multiple ways with fewer adverse side effects than Allopurinol. Sarvepalli found that febuxostat was effective in reducing UA levels in CKD stage IIIA to stage VD without any adverse events (AEs) and no cardiac-related events, which suggest that febuxostat could be a good choice for the treatment of HUA and gout in advanced CKD patients (13). Previous studies have confirmed that febuxostat is effective and safe in patients with advanced CKD and ESRD (14-16). Recently, Choi retrospectively analyzed 62 HUA patients with hemodialysis (HD) and peritoneal dialysis (PD), sUA decreased significantly after febuxostat treatment, and only 2 stopped febuxostat due to its adverse effects. However, the study included only 17 patients on PD (17). Research on febuxostat administration in patients undergoing PD is still insufficient. In this study, we aimed to investigate the safety and efficacy as well as effect on residual renal function (RRF) of febuxostat in patients receiving PD and to provide some clinical experience for HUA treatment in PD patients. We present the following article in accordance with the STROBE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-628/rc).
Methods
Patients
This is a retrospective single-arm cohort study conducted at the Peritoneal Dialysis Center of Ningbo First Hospital involving HUA patients with PD who received febuxostat treatment from September 2016 to November 2020. Our study complied with the Declaration of Helsinki (as revised in 2013) and was approved by the Ningbo First Hospital Institutional Review Board (No. 2021RS002). The requirement for informed consent was waived due to the retrospective nature of the study.
During the study period, 191 patients underwent PD at this hospital. Among these patients, 98 were administrated at least 1 dose of febuxostat therapy and 84 were administrated for over a period of 3 months and were eventually included in our study. The inclusion criteria were as follows: (I) above 18 years old at the initiation of PD, (II) a follow-up of more than 1 month, and (III) with HUA (sUA ≥420 µmol/L in males and UA ≥360 µmol/L in females). We excluded patients with the following criteria: (I) history of allergy to febuxostat, (II) taking any of UA-lowering drugs within 1 month before the index date, (III) changing to HD, (IV) kidney transplantation, (V) liver dysfunction [aspartate aminotransferase (AST) and/or alanine aminotransferase (ALT) levels greater than 2 times of the upper normal limit], and (VI) death.
At the time of febuxostat administration, the demographic data, underlying disease, medications, comorbidities, and laboratory data were collected. The sUA levels were examined at 1, 3, 6, and 12 months after the febuxostat initiation. At the same time, complete blood count and liver function, blood creatinine, blood urea nitrogen, 24 hours residual urine volume, urine creatinine, urine urea nitrogen and other biochemical indexes were determined by hematology analyzer and automatic biochemical analyzer. Clinical information and any AEs associated with febuxostat were acquired from medical records. According to the “Practice Guidelines for Diagnosis and Treatment of Hyperuricemia in Kidney Diseases in China”, the standard of sUA is controlled at <360 µmol/L in PD patients (18). Moreover, RRF was evaluated by estimating glomerular filtration rate (GFR), which was calculated by the mean creatinine and urea clearance (19,20) and examining the renal component of Kt/V urea (21).
Statistical analysis
The software GraphPad InStat version 5.0 (GraphPad Inc., San Diego, CA, USA) was used to perform statistical analysis and data graphics. The count data were expressed as counts and percentages. We applied paired t-test or Wilcoxon signed-rank test to conduct statistical analysis. Values were expressed as means ± standard deviation (SD). Results were considered statistically significant if P<0.05.
Results
The baseline characteristics of patients undergoing PD
The clinical baseline characteristics of patients undergoing PD at the time of febuxostat administration are shown in Table 1. The mean age of patients was 55.18±14.2 (age range, 25 to 89) years and the ratio of male to female was 51:33. In our study, 60 patients took febuxostat 20 mg/day (71.43%), accounting the largest number of patients, and 24 took febuxostat 40 mg/day (28.57%). All 84 patients were administered febuxostat for over 3 months during a regular follow-up from January 2018 to November 2020, including 39 cases for over 6 months and 26 cases for over 12 months. Regarding underlying renal disease of ESRD, the most disease was unknown (46.43%), followed by diabetic kidney disease (21.43%), and primary glomerulonephritis (19.05%). Regarding the comorbid disease, hypertension was the most common comorbidity (86.9%), followed by diabetes (25%), and CVD (21.43%). There were no significant differences in clinical baseline variables between febuxostat 20 and 40 mg/day group (P>0.05).
Table 1
| Characteristics | All patients (n=84) | Febuxostat 20 mg/day (n=60) | Febuxostat 40 mg/day (n=24) |
|---|---|---|---|
| Age (years) | 55.18±14.20 | 56.55±14.49 | 51.75±13.11 |
| Gender, male | 51 (60.71) | 33 (55.00) | 18 (75.00) |
| Follow-up duration (months) | 9.64±9.63 | 10.72±9.54 | 6.95±9.51 |
| SBP (mmHg) | 135.5±18.62 | 134.7±17.77 | 137.7±20.83 |
| DBP (mmHg) | 79.46±10.1 | 77.98±10 | 83.17±9.55 |
| BMI (kg/m2) | 22.51±3.24 | 22.23±3.11 | 23.22±3.53 |
| Causes of ESRD | |||
| Diabetic nephropathy | 18 (21.43) | 13 (21.67) | 5 (20.83) |
| IgA nephropathy | 6 (7.14) | 4 (6.67) | 2 (8.33) |
| Primary glomerulonephritis | 16 (19.05) | 11 (18.33) | 5 (20.83) |
| Urate nephropathy | 5 (5.95) | 4 (6.67) | 1 (4.16) |
| Other or unknown | 39 (46.43) | 28 (46.67) | 11 (45.83) |
| Comorbidities | |||
| Hypertension | 73 (86.90) | 53 (88.33) | 20 (83.33) |
| Diabetics | 21 (25.00) | 16 (26.67) | 5 (20.83) |
| CVD | 18 (21.43) | 15 (25.00) | 3 (12.5) |
| Cerebrovascular disease | 8 (9.52) | 4 (6.67) | 4 (16.67) |
| Gout | 8 (9.52) | 6 (10.00) | 2 (8.33) |
| Blood urea nitrogen (mmol/L) | 18.68±5.88 | 19.21±6.24 | 17.37±4.73 |
| Serum creatine (μmol/L) | 712.6±288 | 725.3±316.8 | 680.9±201.2 |
| sUA (μmol/L) | 498.8±81.47 | 487.2±83.31 | 527.8±70.14 |
| eGFR (mL/min/1.73 m2) | 4.94±3.25 | 4.94±3.37 | 4.93±2.98 |
| PD schemes (number of PD sessions per day) | |||
| Two | 13 (16.66) | 10 (16.66) | 3 (12.50) |
| Three | 35 (41.66) | 25 (41.66) | 10 (41.66) |
| Four | 35 (41.66) | 24 (40.00) | 11 (45.83) |
Values presented as mean ± SD or n (%). PD, peritoneal dialysis; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; ESRD, end-stage renal disease; IgA, immunoglobulin A; CVD, cardiovascular disease; sUA, serum uric acid; eGFR, estimated glomerular filtration rate; SD, standard deviation.
Safety of febuxostat in patients on PD
The AEs involving febuxostat administration are summarized in Table 2. There were 17 (20.24%) AEs associated with febuxostat treatment in our study. A total of 4 patients experienced gout attacks during the first month after febuxostat initiation. Of the 4 patients, 2 (3.33%) were taking febuxostat 20 mg/day and 2 (8.33%) were taking febuxostat 40 mg/day. A total of 5 patients (5.95%) withdrew from our study. The reasons for case withdrawal included: (I) liver dysfunction (AST levels greater than 3 times of the upper normal limit); (II) experiencing platelets below 5×109/L; (III) blurring of vision; (IV) developing leukopenia (white blood cell count lower than 2×109/L); and (V) acute coronary syndrome (a patient with coronary heart disease history). The febuxostat dose when the AEs occurred was 40 mg/day in 6 cases (25%) and 20 mg/day in 11 cases (18.33%), demonstrating that the daily dose of 40 mg was associated with higher AEs compared with the daily dose of 20 mg in patients undergoing PD.
Table 2
| Febuxostat AE | Febuxostat 20 mg/day (n=60) | Febuxostat 40 mg/day (n=24) |
|---|---|---|
| Total number | 11 (18.33) | 6 (25.00) |
| Liver function abnormalities | 2 | 0 |
| Skin rash | 1 | 1 |
| Nausea | 2 | 2 |
| Leukocytopenia | 1 | 0 |
| Thrombocytopenia | 1 | 1 |
| blurring of vision | 1 | 0 |
| Gout attacks | 2 | 2 |
| Diarrhea | 0 | 0 |
| acute coronary syndrome | 1 | 0 |
Values presented as n (%) or n. AE, adverse event; PD, peritoneal dialysis.
Efficacy of febuxostat in patients on PD
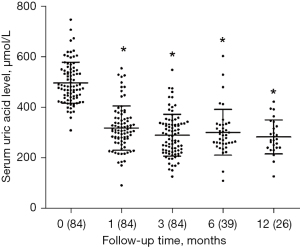
The sUA levels during the 12 months after febuxostat administration are presented in Figure 1. Compared with the baseline level, the mean UA level was markedly reduced at 1 month after febuxostat treatment (320.2±87.27 vs. 498.8±81.47 µmol/L, P<0.0001) and at 3 months (291.6±82.66 vs. 498.8±81.47 µmol/L, P<0.0001) and was subsequently maintained at a significantly low level for 12 months. Of the 84 cases, 72 (85.71%) achieved the target sUA concentration lower than 360 µmol/L at 1 month after febuxostat treatment and 78 cases (92.86%) achieved sUA <360 µmol/L at 3 months. These obvious reductions in sUA level at 1, 3, and 6 months were observed in both the febuxostat 20 and 40 mg/day groups.
Febuxostat effects on RRF in patients with PD
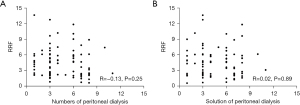
The RRF was evaluated by the renal component Kt/V and the GFR. The RRF of patients with PD before and after febuxostat administration are displayed in Table 3. Assessing RRF by renal component Kt/V revealed that there were no differences in RRF between the two groups before and after febuxostat treatment (P>0.05). As presented in Table 3, evaluating RRF by GFR yielded the same results. There was no association detected between RRF and the number of undertaken dialyses (R=−0.13; P=0.25) after treatment. There was no association between RRF and solution of PD after treatment (R=0.02; P=0.89) (Figure 2).
Table 3
| Project | RRF assessed by Kt/V | RRF assessed by GFR | |||||
|---|---|---|---|---|---|---|---|
| Febuxostat 20 mg/day | Febuxostat 40 mg/day | P value | Febuxostat 20 mg/day | Febuxostat 40 mg/day | P value | ||
| Before treatment | 0.91±0.83 | 0.9±0.51 | 0.46 | 4.95±3.37 | 4.94±2.99 | 0.62 | |
| After treatment | 0.82±0.75 | 0.88±0.61 | 0.18 | 4.41±2.76 | 5.27±2.82 | 0.095 | |
Data presented as mean ± SD. RRF, residual renal function; PD, peritoneal dialysis; Kt/V, K: dialyzer clearance of urea, t: dialysis time, V: volume of distribution of urea; GFR, glomerular filtration rate; SD, standard deviation.
Discussion
In our study, we investigated the safety, efficacy, and effect of febuxostat on RRF in patients undergoing PD and found that febuxostat was a safe and effective urate-lowering drug and might not impair residual kidney function in these patients.
Although febuxostat was reported to be prescribed at unchangeable doses in patients with mild and moderate renal impairment (14,22-24), the proper doses in patients undergoing PD have not been well established. There have only been a handful of studies conducted on the safety and efficacy of febuxostat administration on patients undergoing dialysis (17,25). Recently, Choi et al. retrospectively analyzed 62 dialysis patients with HUA, including 17 PD patients. After febuxostat treatment at a dose of 40–80 mg/day for 3 months, sUA level was significantly reduced and the compliance rate was 87.1%, and UA remained at a low level in the following 12 months (17). In our study, 71.43% of patients received febuxostat at a dose of 20 mg/day, and approximately 28.57% received 40 mg/day, effectively controlling their UA levels. With 1 month of febuxostat administration, the mean UA level was markedly reduced and the decreased UA concentration was retained for more than 12 months in PD patients, demonstrating that febuxostat might be effective for patients on PD.
Many previous studies have reported no serious AEs of febuxostat treatment, and liver function injury was shown to be a common side effect in CKD and dialysis patients (22,23,26). In our study, liver dysfunction was also common AE, but all cases returned to normal within 1 month without taking febuxostat. Gout attacks were observed in 3.33% of patients receiving febuxostat 20 mg once a day after 1 month of treatment, which was lower than that in patients (8.33%) receiving 40 mg once day and that in a study (17). In the Cardiovascular Safety of Febuxostat and Allopurinol in Patients with Gout and Cardiovascular Morbidities (CARES) trial (a double-bind, multicenter clinical trial), patients with gout and major cardiovascular conditions who were prescribed febuxostat had higher all-cause mortality and cardiovascular mortality than those who were administered allopurinol (27). Ghang et al. estimated the data from CARES study and revealed that there were more deaths caused by discontinuation of febuxostat, suggesting that febuxostat discontinuation might be a possible risk factor in all-cause mortality and cardiovascular mortality (28). In our study, there was only 1 patient with a cardiovascular comorbidity who encountered unstable angina with coronary revascularization. In the Febuxostat Versus Placebo Randomized Controlled Trial Regarding Reduced Renal Function IN Patients with Hyperuricemia (FEATHER) study (a randomized, double-bind, placebo-controlled clinical trial), there were no differences in cardiovascular disorders, all-cause mortality, and stroke between stage 3 CKD patients with febuxostat and placebo until week 108 of treatment (29). More studies are needed to confirm the effect of febuxostat on all-cause and cardiovascular mortality in patients with PD. The appropriate dose of febuxostat in patients with PD has not been well established. In our study, 6 (25.00%) of patients taking 40 mg/day experienced AEs and 11 (18.33%) receiving 20 mg/day experienced AEs. Thus, we recommend that febuxostat at an initial dose of 20 mg/day might be safe in patients undergoing PD.
The RRF was evaluated by the renal component Kt/V and the GFR. The RRF plays vital role in ESRD patients, with benefits beyond contributing to achieve adequacy targets. Several studies have demonstrated that RRF preservation is associated with lower risk of death in patients with PD therapy (30-32), an improvement of left ventricular hypertrophy (33) and blood pressure (34), decreased sUA and phosphate levels (32), and lower incidence of peritonitis (35). Renal Kt/V (r-Kt/V) is recommended as an indicator for timing of PD initiation. Kuhlmann et al. demonstrated r-Kt/V falling under a threshold of 2.0/week may indicate inadequate renal toxin excretion (36). In our study, we could not find any association between RRF and the number of undertaken dialyses. Moreover, there was no association between RRF and solution of PD after treatment. The effects of PD modalities on RRF are controversial and more clinical trials are needed to confirm them (37). We revealed that taking febuxostat to decrease sUA was safe and efficient, and the beneficial action did not deteriorate RRF in patients with PD.
There were some limitations to our study. Firstly, it was a retrospective study and the number of cases included was small. Some clinical information, such as AEs, may have been underestimated or missed. A larger sample size, multicenter, prospective randomized controlled trial is needed to further investigate the efficacy and safety of febuxostat in patients undergoing PD. Secondly, since there were only 84 patients who had been treated by febuxostat at the single center in our study, the results cannot be generalized to all patients undergoing PD. Thirdly, patients had been administrated different medications before febuxostat initiation due to having comorbidities such as diabetes, hypertension, and cardiac diseases. It is difficult to attribute changes to a single variable such as febuxostat.
Conclusions
In conclusion, febuxostat is safe and efficient in patients undergoing PD and may not impair RRF. Febuxostat at dose of 20 mg/day may be an appropriate dose for patients undergoing PD.
Acknowledgments
Funding: This study was supported by the Project of Medical and Health Technology Program in Zhejiang Province (No. 2022508312).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-628/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-628/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-628/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study complied with the Declaration of Helsinki (as revised in 2013) and was approved by the Ningbo First Hospital Institutional Review Board (No. 2021RS002). The requirement for informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Harrison R. Structure and function of xanthine oxidoreductase: where are we now? Free Radic Biol Med 2002;33:774-97. [Crossref] [PubMed]
- Sautin YY, Johnson RJ. Uric acid: the oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids 2008;27:608-19. [Crossref] [PubMed]
- Becker MA, Schumacher HR, Espinoza LR, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther 2010;12:R63. [Crossref] [PubMed]
- Bandaru P, Shankar A. Association between Serum Uric Acid Levels and Diabetes Mellitus. Int J Endocrinol 2011;2011:604715. [Crossref] [PubMed]
- Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med 2008;359:1811-21. [Crossref] [PubMed]
- Mallat SG, Al Kattar S, Tanios BY, et al. Hyperuricemia, Hypertension, and Chronic Kidney Disease: an Emerging Association. Curr Hypertens Rep 2016;18:74. [Crossref] [PubMed]
- Ndrepepa G. Uric acid and cardiovascular disease. Clin Chim Acta 2018;484:150-63. [Crossref] [PubMed]
- Ohya M, Shigematsu T. A new xanthine oxidase inhibitor: the uric acid reduction and additional efficacy in CKD patients. Clin Exp Nephrol 2014;18:835-6. [Crossref] [PubMed]
- Madero M, Sarnak MJ, Wang X, et al. Uric acid and long-term outcomes in CKD. Am J Kidney Dis 2009;53:796-803. [Crossref] [PubMed]
- Petreski T, Bevc S, Ekart R, et al. Hyperuricemia and long-term survival in patients with chronic kidney disease undergoing hemodialysis. Clin Nephrol 2017;88:69-72. [Crossref] [PubMed]
- Xiang S, Zhang X, Xie X, et al. High serum uric acid level is a mortality risk factor in peritoneal dialysis patients: a retrospective cohort study. Nutr Metab (Lond) 2019;16:52. [Crossref] [PubMed]
- Grabowski BA, Khosravan R, Vernillet L, et al. Metabolism and excretion of 14C febuxostat, a novel nonpurine selective inhibitor of xanthine oxidase, in healthy male subjects. J Clin Pharmacol 2011;51:189-201. [Crossref] [PubMed]
- Sarvepalli PS, Fatima M, Quadri AK, et al. Study of therapeutic efficacy of febuxostat in chronic kidney disease stage IIIA to stage VD. Saudi J Kidney Dis Transpl 2018;29:1050-6. [Crossref] [PubMed]
- Juge PA, Truchetet ME, Pillebout E, et al. Efficacy and safety of febuxostat in 73 gouty patients with stage 4/5 chronic kidney disease: A retrospective study of 10 centers. Joint Bone Spine 2017;84:595-8. [Crossref] [PubMed]
- Kim SH, Lee SY, Kim JM, et al. Renal safety and urate-lowering efficacy of febuxostat in gout patients with stage 4-5 chronic kidney disease not yet on dialysis. Korean J Intern Med 2020;35:998-1003. [Crossref] [PubMed]
- Lim DH, Oh JS, Ahn SM, et al. Febuxostat in Hyperuricemic Patients With Advanced CKD. Am J Kidney Dis 2016;68:819-21. [Crossref] [PubMed]
- Choi SY, Choi SW, Lee S, et al. Efficacy and tolerability of febuxostat in gout patients on dialysis. Intern Med J 2021;51:348-54. [Crossref] [PubMed]
- Multi-Disciplinary Expert Task Force on Hyperurice. Practice Guidelines for Diagnosis and Treatment of Hyperuricemia in Kidney Diseases in China (2017 Edition). National Medical Journal of China 2017;97:1927-36.
- Chen CH, Perl J, Teitelbaum I. Prescribing high-quality peritoneal dialysis: The role of preserving residual kidney function. Perit Dial Int 2020;40:274-81. [Crossref] [PubMed]
- Brown EA, Blake PG, Boudville N, et al. International Society for Peritoneal Dialysis practice recommendations: Prescribing high-quality goal-directed peritoneal dialysis. Perit Dial Int 2020;40:244-53. [Crossref] [PubMed]
- Moeinzadeh F, Naeini EK, Mortazavi M. Allopurinol Effects on Residual Renal Function in End-Stage Renal Disease Patients Undergoing Peritoneal Dialysis: Randomized Controlled Trial. J Res Pharm Pract 2019;8:189-95. [Crossref] [PubMed]
- Shibagaki Y, Ohno I, Hosoya T, et al. Safety, efficacy and renal effect of febuxostat in patients with moderate-to-severe kidney dysfunction. Hypertens Res 2014;37:919-25. [Crossref] [PubMed]
- Tsuruta Y, Mochizuki T, Moriyama T, et al. Switching from allopurinol to febuxostat for the treatment of hyperuricemia and renal function in patients with chronic kidney disease. Clin Rheumatol 2014;33:1643-8. [Crossref] [PubMed]
- Tanaka K, Nakayama M, Kanno M, et al. Renoprotective effects of febuxostat in hyperuricemic patients with chronic kidney disease: a parallel-group, randomized, controlled trial. Clin Exp Nephrol 2015;19:1044-53. [Crossref] [PubMed]
- Horikoshi R, Akimoto T, Inoue M, et al. Febuxostat for hyperuricemia: experience with patients on chronic hemodialysis treatment. Clin Exp Nephrol 2013;17:149-50. [Crossref] [PubMed]
- Sakai Y, Otsuka T, Ohno D, et al. Febuxostat for treating allopurinol-resistant hyperuricemia in patients with chronic kidney disease. Ren Fail 2014;36:225-31. [Crossref] [PubMed]
- White WB, Saag KG, Becker MA, et al. Cardiovascular Safety of Febuxostat or Allopurinol in Patients with Gout. N Engl J Med 2018;378:1200-10. [Crossref] [PubMed]
- Ghang B, Ahn SM, Kim J, et al. Discontinuing febuxostat might cause more deaths than continuing febuxostat: the untold story from the CARES trial. Rheumatology (Oxford) 2020;59:1439-40. [Crossref] [PubMed]
- Kimura K, Hosoya T, Uchida S, et al. Febuxostat Therapy for Patients With Stage 3 CKD and Asymptomatic Hyperuricemia: A Randomized Trial. Am J Kidney Dis 2018;72:798-810. [Crossref] [PubMed]
- Rocco M, Soucie JM, Pastan S, et al. Peritoneal dialysis adequacy and risk of death. Kidney Int 2000;58:446-57. [Crossref] [PubMed]
- Bargman JM, Thorpe KE, Churchill DN. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol 2001;12:2158-62. [Crossref] [PubMed]
- Marrón B, Remón C, Pérez-Fontán M, et al. Benefits of preserving residual renal function in peritoneal dialysis. Kidney Int Suppl 2008;S42-51. [Crossref] [PubMed]
- Wang AY, Wang M, Woo J, et al. A novel association between residual renal function and left ventricular hypertrophy in peritoneal dialysis patients. Kidney Int 2002;62:639-47. [Crossref] [PubMed]
- Menon MK, Naimark DM, Bargman JM, et al. Long-term blood pressure control in a cohort of peritoneal dialysis patients and its association with residual renal function. Nephrol Dial Transplant 2001;16:2207-13. [Crossref] [PubMed]
- John MM, Gupta A, Sharma RK, et al. Impact of residual renal function on clinical outcome and quality of life in patients on peritoneal dialysis. Saudi J Kidney Dis Transpl 2017;28:30-5. [Crossref] [PubMed]
- Kuhlmann MK, Heckmann M, Riegel W, et al. Evaluation of renal Kt/V as a marker of renal function in predialysis patients. Kidney Int 2001;60:1540-6. [Crossref] [PubMed]
- Li T, Wilcox CS, Lipkowitz MS, et al. Rationale and Strategies for Preserving Residual Kidney Function in Dialysis Patients. Am J Nephrol 2019;50:411-21. [Crossref] [PubMed]
(English Language Editor: J. Jones)


