
How a very large sarcomatoid lung cancer was efficiently managed with lattice radiation therapy: a case report
Introduction
The management of large and unresectable tumors represents an open and concerning issue in the palliative setting. Advanced cancer patients experience a significant burden of symptoms due to the organ compression and infiltration caused by the tumor mass. Since the patients are often fragile due to the advanced disease stage, the therapeutic strategies should be as conservative as possible, to relieve symptoms, preserve quality of life, and avoid excessive toxicity (1).
Considering the unsatisfying results of systemic therapy in patients with bulky radiotherapy (RT) and in particular stereotactic body radiotherapy (SBRT) represents an appealing option. However, due to the high dose delivered in so few fractions, SBRT is usually reserved for smaller lesions (5 cm of maximum diameter) to avoid the risk of severe toxicity, unacceptable for a palliative treatment (2). The standard palliative irradiation is performed with low doses, i.e., 20 Gy/5 fractions or 30 Gy/10 fractions, that are unable to reach a satisfying control of large tumors. In this context, lattice radiation therapy (LRT) may represent an interesting strategy to increase response by delivering very high doses, without added toxicity to the organs at risk (OAR).
LRT is a spatially fractionated radiation therapy (SFRT) technique, based on a non-homogeneous dose distribution in the target with concurrent OAR sparing (3). It consists of a 3D volumetric configuration of the 2D GRID therapy, and it is based on a 3D array that is created inside the target lesion. This array presents high dose areas called vertices (hot spots), separated by lower dose regions called valleys (periphery) (4). The possibility to deliver ablative doses increases the probability of tumor shrinkage, with a durable response. In addition, the heterogeneous dose gradient generated in the treated volume is hypothesized to improve host immune system response against neoplastic cells both in irradiated and non-irradiated sites (5,6).
In this case report, we show and discuss the LRT treatment of a patient with a symptomatic unresectable sarcomatoid lung cancer, a very radioresistant tumor, commonly unresponsive to systemic therapy (7). We present the following case in accordance with the CARE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-246/rc).
Case presentation
All procedures performed in this report were in accordance with the ethical standards of our institutional research committee and with 2013 revised Helsinki declaration. The patient signed a written informed consent for the publication of this paper and for any associated images. A copy of the written consent is available for review by the editorial office of this journal. In May 2020 a 69-year-old male patient (ECOG 1) was admitted to our hospital complaining of severe sacral pain and mild dyspnea. The emergency department radiograph displayed a large opacity (T) in the left lung. The following chest computed tomography (CT) confirmed the sacroiliac lesion (3.8 cm × 3.8 cm) and a large mass (diameters: 11 cm × 12 cm) surrounding and markedly compressing the main left bronchus. In addition, several enlarged bilateral hilar and mediastinal nodes were detected, as well as an analogous new formed lesion close to the gastro-splenic space, another infiltrating the left sacroiliac muscles, and other lesion infiltrating the sacrum. The staging positron emission tomography/CT (PET/CT) concurred with a widespread disease and the sequent endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA), allowed to obtain a sample for cytological analysis, immunohistochemistry, and the tumor proportion score (TPS). These confirmed the diagnosis of a sarcomatoid lung carcinoma PD-L1 TPS >50%, a very aggressive and radioresistant cancer. The disease was staged IV, cT4N3M1c. After a palliative dose of radiotherapy on the sacrum to relieve the back pain (4 Gy × 5 fractions with positive antalgic response and lesion stability), 6 cycles of chemotherapy with carboplatin + paclitaxel were administered Due to primary and mediastinal nodes progression, a switch to nivolumab was made. After 6 cycles, a new progression of disease (PD) leads to third line therapy with gemcitabine + carboplatin (6 cycles). In September 2021, with the patient complaining of worsening dyspnea (ECOG 2), a new CT showed a further PD with a marked increase of the T (19 cm × 16 cm). A fourth line of systemic therapy with vinorelbine was started and the patient was referred to the radiation oncology unit to evaluate the possibility of palliative irradiation (T). Considering the lesion dimension, we shared with the patient and his caregiver the possibility of a LRT instead of a standard palliative RT, explaining all LTR pros and cons in light of available evidence. The patient accepted. From the 10th to 22nd of September 2021, the patient underwent an LRT treatment with the delivery of 55 Gy on the vertices and 20 Gy on the periphery of the volume in 5 fractions. Since vinorelbine is a known radiosensitiser, its administration was suspended during LTR. During the treatment, the patient experienced a G1 asthenia and a G1 esophagitis, quickly relieved with Esifal stick 10 mL BID—a food supplement based on hyaluronic acid, magnesium alginate and keratin, with licorice and plantain extract—and domperidone 10 mg bid (Figure 1 for the timeline).
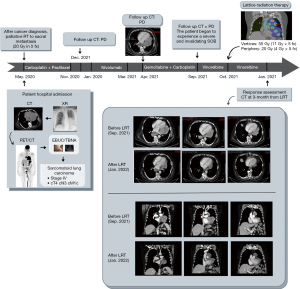
After LRT, the vinorelbine was restarted and the patient was evaluated weekly in the first month and monthly in the second and third months, with no toxicity reported. At 3 months, follow up CT revealed a drastic and unexpected shrinkage of the primary lesion (diameters of 8 cm × 4 cm) and of mediastinal nodes, and the patient reported a noticeable improvement in subjective well-being (ECOG 1) and in his daily life.
As of the last follow up, date of April 30, 2022, the patient was continuing the vinorelbine. He was clinically stable (ECOG 1) and the 6-month CT confirmed the stable disease, with the persistence of the lung lesion response.
Discussion
A treatment planning CT, with and without contrast enhancement, was performed with a slice thickness of 3 mm. For patient’s set up, a Wing Board was used during planning and treatment delivery. The time from planning CT to first fraction was of 8 days. All quality assurance procedures were strictly followed. The LRT treatment was delivered with an IGRT-VMAT technique—7 Arcs—in five daily fractions over one week using a Varian Linac (TrueBeamSN4011). We used 6 MV flattening filter free photon x. For the treatment planning, we used Eclipse treatment planning system version 13.7 (TPS) (Varian Medical Systems, Palo Alto, CA) and the AAA algorithm. Patient’s set up was daily assessed by CBCT. The mean patient time on couch was 20 min.
With reference to the Lattice framework, vertices were separated by 3 cm in the axial plane. The center to-center vertex distance was of 6 cm (4.5 cm edge to edge) in orthogonal axes, and of 3√2 cm along the diagonal axes (spatial distance). We followed S. Luis’ experience and we placed the vertices as in the LITE SABR M1 trial. The Gross Tumor Volume (GTV) is the observable extent of tumor growth. To have no more than 16–18 vertices belonging to the GTV and not to overdose the normal tissue, this framework can be changed (8). In addition to the large mass (T), the GTV included the metastatic intrathoracic lymph nodes adjacent to large mass, since they could be reasonably considered as a single bulky target with the T. Given the GTV and the framework, an in-house software script, developed in MATLAB, suggested the maximum number of vertices that could be generated inside the GTV, and how to optimally place the vertices grid. During lattice framework creation, vertices must not be located less than 1.5 cm of any OARs. We generated 17 vertices (vertex diameter =1.5 cm) inside the mass, for a total volume of 26.5 cc.
We added an isotropic 8 mm to GTV to create the Planning Target Volume (PTV), which accounts for any variation of patients’ position due to organ motion or other movements. We prescribed a dose of 20 Gy in 5 fractions to the 95% of PTV volume (V95%), with 17 simultaneously integrated high-dose boosts in the vertices. Despite the dose reached in the vertices, the dose fall-off outside the PTV was the same of a uniform IMRT plan. Once that the vertex dose had reached a value greater than the 250% of the PTV 133 prescribed dose, the lattice treatment was considered feasible and was delivered to the patient. In this case, we achieved a 55 Gy in the hotspots (11 Gy × 5 fractions) (see Figure 2). Our dose volume endpoints are reported in Table 1.
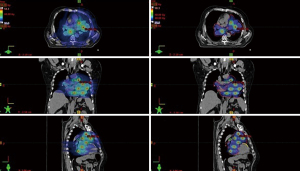
Table 1
| OAR | Dose volume endpoints |
|---|---|
| Total lung | V5 =77.6%; V10 =18.7%; V20 =1.3%; Dmean =9.1 Gy |
| Heart | V5 = 99.8%; V10 =77.9%; V20 =12.6%; Dmean =14 Gy |
| Spinal cord | Dmax =16.9 Gy |
| Esophagus | Dmean =10.6 Gy; Dmax =24.9 Gy |
| Ventricle L | V5 =100%; Dmean =13 Gy |
| Left anterior descending artery | Dmax =23.7 Gy; Dmean =16.3 Gy |
| Left coronary artery | Dmax =23.7 Gy |
| Left atrium | Dmean =14.5 Gy |
| Right atrium | Dmean =9.0 Gy |
Dmax refers to a volume ≤0.035 cc. OAR, organs at risk.
Voluminous neoplastic lesions tend to have an aberrant lymphovascular matrix, with necrotic and hypoxic areas. This limits the chemotherapy efficacy by precluding drug adequate concentration in neoplastic sites. In addition, the typical immunosuppressive microenvironment of these lesions neutralizes the host immune system activity and, consequently, impairs the immunotherapy function (9,10). Although palliative RT appears to be a suitable resource, bulky tumors are usually more radioresistant due to their dimensions and their hypoxic status, moreover, it is not possible to deliver effective high doses with standard regimes, without causing an unacceptable risk of toxicity. This is not justified in a palliative setting that requires above all the preservation of patients’ quality of life and the symptoms control (11). By allowing the delivery of very high doses inside the tumor and concurrently keeping a lower dose at his periphery, LRT can solve this problem and potentially lead to disease response with an acceptable profile of toxicity.
Despite therapeutic potential, few data are currently available on treatment planning and patients’ outcome. Furthermore, many available cases present a hybrid or non-LRT exclusive approach (12,13). For instance, Amendola et al. reported an interesting case series of LRT on NSCLC with a shrinkage of tumor volumes of about 40%. However, they used a hybrid approach because the first LRT fraction (18 Gy on the vertices and 3 Gy on the periphery) was followed by a conventional RT (25/33 daily fractions of 1.8–2 Gy) (12). An analogous hybrid LRT was also used in another case series of stage IIIB–IVA bulky cervical cancers where patient received a LTR (24 Gy on the vertices and 9 Gy on the periphery in 3 fractions), followed by a conventional RT (39.60–45.00 Gy in 1.8 Gy fractions) (13). In addition to its novelty, the fear of delivering an excessive dose with a proper LRT treatment (i.e., a multi-fraction treatment) could be a possible reason why LRT clinical literature is poor. However, it is worth noting that the safety of LRT was confirmed for the first time by the recent publication of the “LITE SABR M1 phase I trial” (8). In this study, 20 patients with bulky lesions were treated with LRT doses of 66.70 Gy in the vertices and 20 Gy in the periphery of the volume in 5 fractions (13.34 and 4 Gy per fraction, respectively). The same group has been carrying out an ongoing phase II clinical trial (NCT04553471) to evaluate the efficacy and the late toxicity of LRT in patients with bulky sarcoma and cancers of the thorax, abdomen, and pelvis.
When our patient was treated, the results of “LITE SABR M1 phase I trial” had not been published yet, therefore, we decided to prudently limit the dose in the hotspots to 55 Gy (11 Gy per fraction) and to 20 Gy in the periphery (4 Gy per fraction). In addition to the considerably objective tumor response, what seems interesting to report is the subjective response stated by the patients in terms of “well-feeling”, which was repeated at each follow-up visit, even after a short time from the end of the LRT.
In addition to the ablative dose delivery that could overcome neoplastic cells radioresistance, some studies suggest that LRT may provoke cancer cells immunogenic death, with the release of many “damage associated molecular patterns” (DAMPs), that prime and strongly enhance host immune system response (14,15). It is hypothesized that the concomitant presence of low dose regions could preserve the residual blood flow and allow the DAMPs circulation, which is essential to trigger antitumor immunity. Thus, LRT could have both an ablative action (local activity) and an immunomodulatory activity (abscopal effect) thanks to the heterogeneous dose distribution (16). Thereby, in addition to a drastic cytoreduction due to high doses, LRT could reengineer the immunosuppressive TME, making it more immunogenic, and consequently starts an anti-tumor immune response both in irradiated sites (bystander effect) and distant ones (abscopal effect) (17).
In light of this, although data on LRT immunomodulatory actions are preliminary and further research is mandatory, this interesting hypothesis may widen LTR from a palliative setting to a curative one, both in bulky localized and widespread disease (18,19).
Despite the considerable tumor response, our report two limitations. First, this is a case report of a single LRT treatment, delivered with the primary aim to provide symptoms relief, and the LRT action on the host immune system was not investigated. Secondly, notwithstanding the registered low toxicity profile of LRT in acute, the data on an eventual LRT chronic toxicity is currently not available in this patient because of a too short follow up.
Conclusions
LRT could represent a valid strategy to obtain a clinically significant tumor response and, consequently, to improve the patient's quality of life without causing treatment-related toxicity. No data is currently available on which could be the best strategy between exclusive LRT and hybrid LRT. As a result, all LRT approach should be positively welcome and investigated. This case report provides another evidence of the clinical value, safety and effectiveness of large tumors management with LRT. Additionally, we provide further data on LRT planning.
Acknowledgments
We would like to express our profound thanks to all the staff of Radiotherapy and Medical Physic Unit of the Azienda USL-IRCCS di Reggio Emilia, Reggio Emilia, Italy.
Funding: This study was partially supported by Italian Ministry of Health – Ricerca Corrente Annual Program 2023.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-246/rc
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-246/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-246/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this study and any accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rodrigues G, Sanatani M. Age and comorbidity considerations related to radiotherapy and chemotherapy administration. Semin Radiat Oncol 2012;22:277-83. [Crossref] [PubMed]
- Allibhai Z, Taremi M, Bezjak A, et al. The impact of tumor size on outcomes after stereotactic body radiation therapy for medically inoperable early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2013;87:1064-70. [Crossref] [PubMed]
- Duriseti S, Kavanaugh J, Goddu S, et al. Spatially fractionated stereotactic body radiation therapy (Lattice) for large tumors. Adv Radiat Oncol 2021;6:100639. [Crossref] [PubMed]
- Wu X, Perez NC, Zheng Y, et al. The Technical and Clinical Implementation of LATTICE Radiation Therapy (LRT). Radiat Res 2020;194:737-46. [Crossref] [PubMed]
- Asur R, Butterworth KT, Penagaricano JA, et al. High dose bystander effects in spatially fractionated radiation therapy. Cancer Lett 2015;356:52-7. [Crossref] [PubMed]
- Kumari S, Mukherjee S, Sinha D, et al. Immunomodulatory Effects of Radiotherapy. Int J Mol Sci 2020;21:8151. [Crossref] [PubMed]
- Li X, Wu D, Liu H, et al. Pulmonary sarcomatoid carcinoma: progress, treatment and expectations. Ther Adv Med Oncol 2020;12:1758835920950207. [Crossref] [PubMed]
- Duriseti S, Kavanaugh JA, Szymanski J, et al. LITE SABR M1: A phase I trial of Lattice stereotactic body radiotherapy for large tumors. Radiother Oncol 2022;167:317-22. [Crossref] [PubMed]
- Karsch-Bluman A, Feiglin A, Arbib E, et al. Tissue necrosis and its role in cancer progression. Oncogene 2019;38:1920-35. [Crossref] [PubMed]
- Hinshaw DC, Shevde LA. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res 2019;79:4557-66. [Crossref] [PubMed]
- O'Donovan A, Morris L. Palliative Radiation Therapy in Older Adults With Cancer: Age-Related Considerations. Clin Oncol (R Coll Radiol) 2020;32:766-74. [Crossref] [PubMed]
- Amendola BE, Perez NC, Wu X, et al. Safety and Efficacy of Lattice Radiotherapy in Voluminous Non-small Cell Lung Cancer. Cureus 2019;11:e4263. [Crossref] [PubMed]
- Amendola BE, Perez NC, Mayr NA, et al. Spatially Fractionated Radiation Therapy Using Lattice Radiation in Far-advanced Bulky Cervical Cancer: A Clinical and Molecular Imaging and Outcome Study. Radiat Res 2020;194:724-36. [Crossref] [PubMed]
- Kanagavelu S, Gupta S, Wu X, et al. In vivo effects of lattice radiation therapy on local and distant lung cancer: potential role of immunomodulation. Radiat Res 2014;182:149-62. [Crossref] [PubMed]
- Jarosz-Biej M, Smolarczyk R, Cichoń T, et al. Tumor Microenvironment as A "Game Changer" in Cancer Radiotherapy. Int J Mol Sci 2019;20:3212. [Crossref] [PubMed]
- Ferini G, Valenti V, Tripoli A, et al. Lattice or Oxygen-Guided Radiotherapy: What If They Converge? Possible Future Directions in the Era of Immunotherapy. Cancers (Basel) 2021;13:3290. [Crossref] [PubMed]
- Cytlak UM, Dyer DP, Honeychurch J, et al. Immunomodulation by radiotherapy in tumour control and normal tissue toxicity. Nat Rev Immunol 2022;22:124-38. [Crossref] [PubMed]
- Jiang L, Li X, Zhang J, et al. Combined High-Dose LATTICE Radiation Therapy and Immune Checkpoint Blockade for Advanced Bulky Tumors: The Concept and a Case Report. Front Oncol 2021;10:548132. [Crossref] [PubMed]
- Boyce-Fappiano D, Damron EP, Farooqi A, et al. Hypofractionated Radiation Therapy for Unresectable or Metastatic Sarcoma Lesions. Adv Radiat Oncol 2022;7:100913. [Crossref] [PubMed]


