
Detection of an EML4-ALK fusion mutation secondary to epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) therapy for lung cancer: a case report
Introduction
Lung cancer has the highest morbidity and mortality of all malignancies worldwide, and approximately 85% of lung cancer patients have non-small cell lung cancer (NSCLC). Furthermore, more than half of the patients are in an advanced stage at diagnosis (1). Identifying the relevant driver genes and selecting the appropriate targeted drugs is vital to improving the prognosis of patients with advanced NSCLC. Epidermal growth factor receptor (EGFR) mutations occur in around 50% of relevant cases and is one of the most common driver genes in lung cancer. The most common gene mutations include EGFR 19del and EGFR 21L858R. In patients with advanced NSCLC and sensitive mutations, administration of EGFR-tyrosine kinase inhibitors (TKIs) has shown significantly better therapeutic effect compared to conventional chemotherapy. Globally, treatment guidelines recommend EGFR-TKIs as the best option for first-line treatment. However, most patients develop drug resistance at around 9–14 months after administration of first-line treatment with first- or second-generation EGFR-TKIs. The most common mechanisms for acquired drug resistance include secondary point mutations (e.g., T790M mutation), bypass activation (e.g., c-MET amplification), human epidermal growth factor receptor 2 (HER2) mutation, and histological type transformation (2,3). The T790M mutation is the most common mechanism for acquired drug resistance (4), occurring in around 60% of cases. The third generation of EGFR-TKIs, such as osimertinib, can overcome the acquired drug resistance caused by the T790M mutation and can achieve a better curative effect on the disease. However, inevitably, drug resistance will re-occur (5). For the third generation of TKIs, drug resistance mechanisms include secondary EGFR point mutation, T790M loss, EGFR amplification, abnormal bypass activation, and histological phenotype transformation. Additionally, in some cases, oncogene fusion may also be a potential mechanism for drug resistance. In the AURA3 study (6), amongst the osimertinib resistant cases, one patient presented with a RET-ERC1 gene fusion and another had a NTRK1-TPM3 gene fusion. The FLAURA study (7) identified one case of ALK gene fusion. Almonertinib, a third generation EGFR-TKI that was independently developed in China, has been shown in the APOLLO study (8) to be safe and effective for use in T790M positive patients with locally advanced or metastatic NSCLC. However, there are few studies examining the mechanisms of resistance developed after almonertinib administration. This current report details a young woman diagnosed with advanced lung adenocarcinoma during pregnancy who had undergone biopsies following treatment with icotinib and almonertinib. An ALK gene rearrangement was identified using dynamic detection, and this is the first case report detailing the detection of an ALK rearrangement serves as a rare molecular mechanism to almonertinib resistance, which contributes to the limited body of literature examining ALK rearrangement as a mechanism of resistance to EGFR-TKIs in advanced EGFR-mutant NSCLC. We present the following article in accordance with the CARE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-744/rc).
Case presentation
The patient is a 35-year-old Han Chinese female, who was 8 months pregnant. She had no history of smoking and had previously been healthy. She underwent a cesarean section on May 13, 2020 and was admitted to our hospital 2 days after the procedure due to an intermittent cough she had experienced for more than half a year. Physical examination showed multiple enlarged lymph nodes palpable on both sides of her neck, which were tough and mildly tender. The patient presented with a relatively large node on the right side of her neck, approximately 2 cm in diameter. She had low breathing sounds in her lower lungs but no wet or dry rale, and no pleural friction sounds were heard. On auxiliary examination there was a tumor marker carcinoembryonic antigen 81.95 ng/mL, soluble cytokeratin 298.60 ng/mL, and neuron-specific enolase 42.43 ng/mL. Others were within the corresponding normal ranges. A contrast-enhanced computed tomography (CT) scan of the chest and abdomen showed changes after the cesarean section. A mass was observed in the inferior lobe of the left lung, with a lobulation approximately 6.5 cm long present at the margin. There were multiple enlarged lymph nodes throughout the body, multiple low-density shadows about 3 cm long in the liver, multiple areas with uneven bone density, and bilateral pleural effusion accompanied with atelectasis of the adjacent lung tissue. There was a small amount of pericardial effusion, and also abdominal effusion. A magnetic resonance imaging (MRI) scan of the head showed no obvious abnormalities. Bone imaging scans found multiple abnormal punctate groupings all over the body, which were determined as multiple bone metastases. Color-guided ultrasound was used to perform a right supraclavicular lymph biopsy and pathological findings revealed adenocarcinoma, exfoliative cell examination from the bilateral pleural fluid identified cancer cells, suggestive of metastatic lung adenocarcinoma. A peritoneal wash was sent for testing during the caesarean delivery, and the results suggested that malignancy could not be excluded. In addition, heterogeneous cells were observed. Genetic testing of the lymph node tissue (by next-generation sequencing) showed an in-frame deletion mutation in EGFR exon 19, with an abundance of 79.00%. The EGFR copy number was amplified (copy number =8.76), while the other gene mutations such as ALK, ERBB2, BRAF, MET, RET, ROS1, KRAS, and PIK3CA were not detected. The primary diagnosis was determined to be left lung adenocarcinoma of State IVB (T3N3M1c), with liver, bilateral pleural, and bone metastasis, in addition to a EGFR exon 19 deletion.
From May 20, 2020, the patient was treated with 0.125 g icotinib hydrochloride, 3 times daily. After 1 month of treatment, partial response (PR) was detected. The chest CT revealed that the mass in the lower lobe of the left lung was reduced by 43%, from 6.5 to 3.7 cm in long diameter. All target lesions shrunk by about 50%. There were no obvious adverse side effects. Subsequently, the patient was regularly re-examined and continuously evaluated as a PR case. In November 2020, the patient presented with enlarged cervical lymph nodes on both sides of the cervical lymph node. A color Doppler ultrasonography showed that the size of the lumps was approximately 2.0 cm × 0.9 cm. Following a re-examination by chest and abdomen enhanced CT, newly enlarged lymph nodes under the axilla were discovered. She was considered to have progressive disease (PD), the progression-free survival (PFS) of using icotinib as first-line treatment was 5 months. An EGFR T790M genetic test was performed on the patient’s blood and the results indicated positive T790M mutation (abundance: 3.36%). As second-line treatment, 110 mg almonertinib was administered once daily. Initial outcome evaluation suggested PR. In April 2021, enlarged cervical lymph nodes were detected. Following a chest and abdomen CT scan, a low-density shadow was observed in the spleen with a high possibility of metastasis. In addition, there were increased and enlarged lymph nodes throughout the body, as well as increased and enlarged liver metastases. A head MRI scan found no signs of brain metastasis. The outcome was evaluated as PD [lung lesion: stable disease (SD); metastasis: PD]. As second-line treatment, almonertinib PFS was 5 months. Genetic testing in another biopsy of the left supraclavicular lymph nodes showed p.E746-A750del in-frame deletion mutation in the EGFR exon 19, with an abundance of 43.20%. There was amplification of the EGFR copy number (copy number =2.4). A gene rearrangement involving ALK, namely echinoderm microtubule-associated protein-like 4 (EML4)-ALK (E2:A20), with an abundance of 7.36%, was detected. Almonertinib combined with crizotinib was administered as the third-line treatment. At the last follow-up on May 26, 2021, the patient was considered to be in partial remission (PR). The detailed treatment regimen and adverse events of this patient are described in Figure 1. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
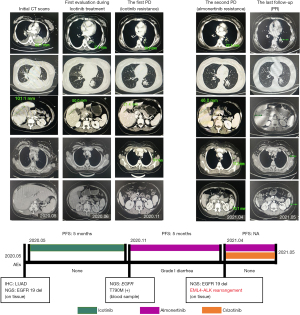
Discussion
The development of targeted therapy has created a breakthrough in the treatment of NSCLC, which has not only significantly prolonged patient survival, but also improved their quality of life. Currently, for mutation positive patients with NSCLC, targeted therapy is the regimen for first-line treatment.
EGFR-TKIs are the standard regimen for first-line treatment in patients with NSCLC of EGFR sensitive mutations. Osimertinib has been approved for second-line treatment in patients with advanced NSCLC who show positive T790M after EGFR-TKI treatment, and for first-line treatment for EGFR-sensitive mutation. Some studies have shown that osimertinib-resistant patients who show positive T790M after undergoing posterior line treatment, the initial L858R mutation is more prone to C797S mutation. Meanwhile, for the initial 19del, T790M loss and activation of the EGFR-independent resistance pathway are more likely to occur, e.g., the most common MET amplification (9). At present, there are few reports on ALK gene rearrangement as the mechanism of osimertinib resistance, which accounts for only 0.13% of the total cases which has been reported (10). Hou et al. performed a retrospective analysis on 7 cases of secondary ALK rearrangement after developing osimertinib resistance (11). The results showed that dual-targeted therapy was beneficial after osimertinib resistance mediated by ALK gene rearrangement had occurred, and suggested that ALK gene rearrangement might be the underlying cause of osimertinib resistance. Many cases of ALK gene rearrangement occurring after osimertinib resistance have been reported (12), and for these cases, positive outcomes were achieved after patients were subsequently treated with ALK-TKIs.
Almonertinib (13,14) is a third generation EGFR-TKI approved for global use. Its design is based on a modification of the structure of osimertinib, whereby the methyl group at the indole ring is replaced by a cyclopropyl group, thus enhancing the selectivity and inhibitory effects on the T790M mutation, and increasing the permeability of the blood-brain barrier. The APOLLO phase II clinical study (8) included a total of 244 patients with advanced NSCLC from China, who were EGFR T790M positive after undergoing first-line treatment with EGFR-TKIs. The results showed positive outcomes and demonstrated the safety of second-line treatment with almonertinib for EGFR T790M positive patients. Second-line treatment with almonertinib resulted in significantly reduced lesions and the progression-free survival was 5 months. Further genetic testing found loss of T790M mutation and EML4-ALK fusion mutation. At present, there are no reports investigation the mechanisms of almonertinib-related drug resistance. This current case report suggests that ALK rearrangement may be a potential cause of almonertinib-related drug resistance.
It should be noted that this patient was pregnant when diagnosed with advanced lung cancer. Yang et al. (15) examined 64 cases of pregnancy complicated with lung cancer and found that 54 patients (84.38%) were confirmed in the middle or late trimester, suggesting that most patients are often misdiagnosed in early pregnancy, and both the gestational age and tumor stage are often advanced at diagnosis, resulting in poor prognosis. A study conducted by Niikawa et al. (16) involving 59 cases with NSCLC, demonstrated that 43 patients had significantly higher estradiol levels in lung cancer tissues compared to normal lung tissues. In addition, for estrogen receptor positive cases, the estradiol level was positively correlated with tumor size and Ki-67 expression level in the tumor tissue.
During pregnancy, the maternal hormone levels may be elevated and immune function compromised, thus accelerating tumor growth. Estrogen receptors can be divided into two subtypes: estrogen receptor α (ERα) and estrogen receptor β (ERβ). ERβ is highly expressed in lung cells and bronchial epithelial cells. Nose et al. (17) evaluated 447 specimens after lung adenocarcinoma surgery and demonstrated that EGFR mutations were associated with a high expression of ERβ in the nucleus. Among the patients studied, 67% were positive for EGFR gene mutation. However, the relationship between estrogen and lung cancer must be explored further in future studies.
The patient in this current case report was diagnosed with lung cancer in her last trimester of pregnancy. Genetic testing indicated an EGFR 19del, and the association of estrogen levels in the body with the lung cancer gene mutation could not be excluded. After multi-line targeted therapies, the patient’s overall survival reached up to 13 months. Therefore, for pregnant patients with lung cancer, early detection and timely treatment may prolong their survival. In addition, during diagnosis and treatment, appropriate treatment regimens should be selected according to the patient’s individual conditions and gestational age.
There were some limitations to this case report. Firstly, the estrogen and progesterone levels were not monitored during pregnancy, so the relationship between estrogen levels and gene mutation cannot be concluded, more research on the association are needed. Secondly, at the time of the patient’s first progression, we only sent the peripheral blood for EGFR T790M gene testing instead of comprehensive genetic testing, the plasma assay of EGFR T790M single-point may insufficient to identify the resistance of EGFR-TKIs, since concurrent driver gene resistance impairs icotinib’s efficacy. And also whether there was an ALK fusion gene rearrangement or not at the first progression remains uncertain. Thirdly, the re-biopsy site only represents a partial pathology of the resistance, and the mechanism of resistance may differ from one site to another.re-biopsy of only one site might not always be appropriate. In this case report, we only sent cervical lymph node tissue samples during the initial diagnosis and subsequent progress, but did not submit lung primary lesion tissue samples. It is not possible to determine whether there is heterogeneity between the lung primary lesion and lymph node samples. The use of NGS to detect ctDNA in blood samples for supplementary biopsy may enhance the credibility and better guide treatment. This highlights the need for comprehensive next generation sequencing (NGS) assays.
Conclusions
This is the first case report detailing the detection of an ALK rearrangement after almonertinib resistance in advanced EGFR-mutant NSCLC, and the combination of ALK TKI therapy and almonertinib may be a viable strategy in this setting. This case suggested that ALK rearrangement may be an underlying mechanism of resistance to almonertinib.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-744/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-744/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Wu Q, Luo W, Li W, et al. First-Generation EGFR-TKI Plus Chemotherapy Versus EGFR-TKI Alone as First-Line Treatment in Advanced NSCLC With EGFR Activating Mutation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front Oncol 2021;11:598265. [Crossref] [PubMed]
- Zhong WZ, Zhou Q, Wu YL. The resistance mechanisms and treatment strategies for EGFR-mutant advanced non-small-cell lung cancer. Oncotarget 2017;8:71358-70. [Crossref] [PubMed]
- Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med 2005;2:e73. [Crossref] [PubMed]
- Leonetti A, Sharma S, Minari R, et al. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer 2019;121:725-37. [Crossref] [PubMed]
- Wu YL, Mok TSK, Han JY, et al. 475O - Overall survival (OS) from the AURA3 phase III study: Osimertinib vs platinum-pemetrexed (plt-pem) in patients (pts) with EGFR T790M advanced non-small cell lung cancer (NSCLC) and progression on a prior EGFR-tyrosine kinase inhibitor (TKI). Ann Oncol 2019;30:ix158. [Crossref]
- Cho BC, Ohe Y, Zhou C. LBA16 - Osimertinib vs comparator EGFR-TKI as first-line treatment for EGFRm advanced NSCLC (FLAURA): Final overall survival analysis. Ann Oncol 2019;30:ix198-ix199. [Crossref]
- Lu S, Wang Q, Zhang G, et al. Abstract CT190: A multicenter, open-label, single-arm, phase II study: The third generation EGFR tyrosine kinase inhibitor almonertinib for pretreated EGFR T790M-positive locally advanced or metastatic non-small cell lung cancer (APOLLO). AACR 2020.
- Wang C, Zhao K, Hu S, et al. Patterns and Treatment Strategies of Osimertinib Resistance in T790M-Positive Non-Small Cell Lung Cancer: A Pooled Analysis. Front Oncol 2021;11:600844. [Crossref] [PubMed]
- Xu H, Shen J, Xiang J, et al. Characterization of acquired receptor tyrosine-kinase fusions as mechanisms of resistance to EGFR tyrosine-kinase inhibitors. Cancer Manag Res 2019;11:6343-51. [Crossref] [PubMed]
- Hou H, Sun D, Zhang C, et al. ALK rearrangements as mechanisms of acquired resistance to osimertinib in EGFR mutant non-small cell lung cancer. Thorac Cancer 2021;12:962-9. [Crossref] [PubMed]
- Batra U, Sharma M, Amrith BP, et al. EML4-ALK Fusion as a Resistance Mechanism to Osimertinib and Its Successful Management With Osimertinib and Alectinib: Case Report and Review of the Literature. Clin Lung Cancer 2020;21:e597-600. [Crossref] [PubMed]
- Nagasaka M, Zhu VW, Lim SM, et al. Beyond Osimertinib: The Development of Third-Generation EGFR Tyrosine Kinase Inhibitors For Advanced EGFR+ NSCLC. J Thorac Oncol 2021;16:740-63. [Crossref] [PubMed]
- Yang JC, Camidge DR, Yang CT, et al. Safety, Efficacy, and Pharmacokinetics of Almonertinib (HS-10296) in Pretreated Patients With EGFR-Mutated Advanced NSCLC: A Multicenter, Open-label, Phase 1 Trial. J Thorac Oncol 2020;15:1907-18. [Crossref] [PubMed]
- Yang L, He YT, Kang J, et al. Clinical features and intervention timing in patients with pregnancy-associated non-small-cell lung cancer. J Thorac Dis 2021;13:4125-36. [Crossref] [PubMed]
- Niikawa H, Suzuki T, Miki Y, et al. Intratumoral estrogens and estrogen receptors in human non-small cell lung carcinoma. Clin Cancer Res 2008;14:4417-26. [Crossref] [PubMed]
- Nose N, Sugio K, Oyama T, et al. Association between estrogen receptor-beta expression and epidermal growth factor receptor mutation in the postoperative prognosis of adenocarcinoma of the lung. J Clin Oncol 2009;27:411-7. [Crossref] [PubMed]
(English Language Editor: J. Teoh)


