
New hope for patients with BRAF V600E-mutant anaplastic thyroid cancer: lights and shadows
Patients with anaplastic thyroid cancer (ATC) are all considered stage IV in the American Joint Committee on Cancer staging manual, due to the poor prognosis of this undifferentiated form of follicular-cell derived thyroid cancer. Standard therapeutic approaches (such as surgery and radioiodine therapy) are not effective. The historical median survival from diagnosis is about 5 months (ranging from 3 to 8 months if disease is initially confined to the neck). Half of all deaths are attributable to upper airway obstruction, while the rest stem from complications of distant disease or therapies (1,2).
Until recently, cytotoxic chemotherapy was the primary treatment for metastatic ATC, despite low response rates and significant toxicity. Recommended regimens included paclitaxel or doxorubicin, alone or in combination with other drugs and administered weekly or every 3–4 weeks. Multimodal approaches involving external beam radiotherapy resulted in improved, but still poor overall outcomes. Evidence-based guidance is still lacking for decisions on second-line therapy. Response rates with traditional chemotherapy are about 15% (1,2).
The combination of the BRAF-inhibitor dabrafenib with the mitogen-activated protein kinase kinase (MEK) inhibitor trametinib has recently been approved by the United States Food and Drug Administration (FDA) for the treatment of locally advanced or metastatic BRAF V600E-mutant ATC with no other locoregional treatment options. Approval was based on data from a phase II trial, in which the overall response rate (ORR) was 69% and the treatment was well-tolerated (3). The 12-month response rate was 90% (3). In a neoadjuvant setting, therapy with dabrafenib and trametinib was demonstrated to prolong overall survival (OS) in BRAF-mutant locally advanced patients, as compared with historical data (rates at 6 months and 1 year: 100% and 83%, respectively) (4). Therapy with dabrafenib and trametinib dramatically changed the natural history of patients with BRAF V600E-mutant ATC and is now recommended by clinical practice guidelines (5-7).
Subbiah and colleagues recently published an updated analysis from the Rare Oncology Agnostic Research (ROAR) open-label, nonrandomized, phase II basket study (NCT02034110) (8). While the first report (3) included only 16 patients (15 from the primary cohort and 1 from the extension cohort), the updated analysis includes the full cohort of 36 patients and about 4 additional years of follow-up [median follow-up: 11.1 (range, 0.9–76.6) months]. All patients were recruited from subspecialty centers with expertise in head and neck or endocrine pathology. At data cutoff, the ORR was 56% (95% confidence interval: 38.1–72.1%). Median progression free survival (PFS) and OS were 6.7 and 14.5 months, respectively. Out of the whole cohort, 34 patients discontinued treatment: 22 due to progressive disease, 6 due to adverse events (pyrexia in most cases, which occurred early during the treatment course), and 6 due to withdrawal.
As the authors point out, these data substantially confirm the clinical benefit of this combination for patients with ATC. These data should be confirmed in larger cohorts, considering some caveats. The rarity and aggressiveness of ATC have limited the development of specific clinical trials. Further trials and enrolment should be encouraged for ATC patients with good clinical performance statuses. This is urgently needed for several reasons.
Not all ATCs are the same disease. Despite their rarity, many variants have been described. These aggressive cancers develop from more differentiated tumors following one or more dedifferentiation events. BRAF and RAS mutations are less common than in differentiated thyroid cancer (DTC). The BRAF V600E mutation is found in 10–50% of ATCs. Additional late mutations, such as TERT promoter and TP53 gene mutations, are frequent and implicated in dedifferentiation, although the precise mechanisms are still unclear. Four genetically distinct ATC types have been identified (9). Type 1 consists almost exclusively of BRAF V600E-mutated ATCs with additional mutations in PIK3CA, AKT1, or ARID2, a genetic profile suggesting that type 1 ATCs probably evolve from papillary thyroid carcinoma (PTC). Type 2 ATCs harbor NRAS mutations plus CCNE1 copy number gains and probably originate from NRAS-mutant follicular thyroid cancers (FTCs). Type 3 ATCs have a higher number of genetic alterations and include those ATCs with defective DNA mismatch repair deriving from MSH2 and MLH1 gene mutations. The genetic profiles of type 3 ATCs suggest that they most likely originate from Hürthle cell carcinoma or from a subset of RAS-mutant FTC. Another mixed group of tumors has loss-of-function alterations in the cell-cycle regulator genes CDKN2A and CDKN2B and genetic features of the other three ATC subtypes (e.g., BRAF mutations, NRAS mutations with PTEN/NF1/RB1 mutations). This group does not seem to derive from a pre-existing DTC (9).
Therefore, only a subset of patients is candidate to receive treatment with dabrafenib and trametinib after genotyping tumor samples. Beyond genetics, it is noteworthy that the ROAR trial also excluded patients with squamoid-differentiated ATC and those who could not swallow pills, thus further reducing the pool of individuals potentially eligible for treatment (dysphagia is a common feature of advanced ATC).
Patients with wild-type BRAF have more limited options (Figure 1). The efficacy of multikinase inhibitors (MKI), such as lenvatinib, in ATC is controversial. An international open-label, multicenter, phase II study that enrolled patients with ATCs to receive lenvatinib was halted for futility (10). Only one patient achieved a partial response (ORR 2.9%). The median PFS was 2.6 months, and OS was 3.2 months.
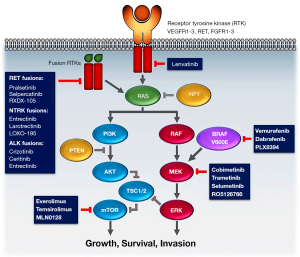
Selective targeted therapies have been proposed for ATC cases with other specific genetic alterations (e.g., pralsetinib or selpercatinib for RET fusions, larotrectinib or entrectinib for NTRK fusion) (11-14) or high tumor mutational burden or microsatellite instability (e.g., pembrolizumab or spartalizumab and combinations of MKIs and immunotherapy) (15-17). These approaches are currently being investigated, though data are scarce (most basket trials include a very small number of ATCs) and there is no regulatory approval for any of these approaches for the specific treatment of ATC. It should also be considered that while targeting a single mutation may be attractive, it may lead to secondary failures or the development of acquired resistance (18), and may not necessarily affect the complex network of genetic and metabolic events that cause cancer initiation and progression (19,20).
It is worth noting that favorable outcomes were obtained in tertiary referral centers that optimized fast-track protocols for ATC patients (21). For example, in 2014, the University of Texas MD Anderson Cancer Center created a dedicated team (the Facilitating Anaplastic Thyroid Cancer Specialized Treatment team) that provided patients with rapid access to multidisciplinary care and tumor molecular testing (22). Indeed, the appropriate management of ATC is time-sensitive and warrants histological confirmation, urgent radiological staging, appropriate evaluation of airways, multidisciplinary planning, and at least BRAF testing, with next-generation sequencing analysis being the preferred approach if available (Figure 2) (5,6). However, these ambitious clinical pathways are still a long way from becoming standard in current clinical practice.
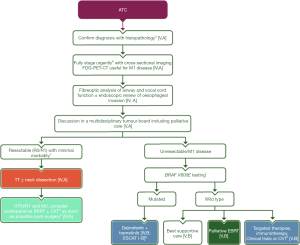
While the prognosis of patients with ATC has considerably improved in the last decade and these improvements are expected to continue into the future, there are still unmet needs that should be addressed by future research. Clinical success also depends on establishing centers of excellence, mapping clinical pathways, and prioritizing ATC patients in order to offer timely and optimal care.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Palliative Medicine. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-603/coif). CD has reported advisory boards for Eisai, Eli Lilly. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Smallridge RC, Ain KB, Asa SL, et al. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid 2012;22:1104-39. [Crossref] [PubMed]
- Cabanillas ME, Zafereo M, Williams MD, et al. Recent advances and emerging therapies in anaplastic thyroid carcinoma. F1000Res 2018;7:eF1000 Faculty Rev-87.
- Subbiah V, Kreitman RJ, Wainberg ZA, et al. Dabrafenib and Trametinib Treatment in Patients With Locally Advanced or Metastatic BRAF V600-Mutant Anaplastic Thyroid Cancer. J Clin Oncol 2018;36:7-13. [Crossref] [PubMed]
- Wang JR, Zafereo ME, Dadu R, et al. Complete Surgical Resection Following Neoadjuvant Dabrafenib Plus Trametinib in BRAFV600E-Mutated Anaplastic Thyroid Carcinoma. Thyroid 2019;29:1036-43. [Crossref] [PubMed]
- Filetti S, Durante C, Hartl D, et al. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol 2019;30:1856-83. [Crossref] [PubMed]
- Filetti S, Durante C, Hartl DM, et al. ESMO Clinical Practice Guideline update on the use of systemic therapy in advanced thyroid cancer. Ann Oncol 2022;33:674-84. [Crossref] [PubMed]
- Bible KC, Kebebew E, Brierley J, et al. 2021 American Thyroid Association Guidelines for Management of Patients with Anaplastic Thyroid Cancer. Thyroid 2021;31:337-86. [Crossref] [PubMed]
- Subbiah V, Kreitman RJ, Wainberg ZA, et al. Dabrafenib plus trametinib in patients with BRAF V600E-mutant anaplastic thyroid cancer: updated analysis from the phase II ROAR basket study. Ann Oncol 2022;33:406-15. [Crossref] [PubMed]
- Pozdeyev N, Gay LM, Sokol ES, et al. Genetic Analysis of 779 Advanced Differentiated and Anaplastic Thyroid Cancers. Clin Cancer Res 2018;24:3059-68. [Crossref] [PubMed]
- Wirth LJ, Brose MS, Sherman EJ, et al. Open-Label, Single-Arm, Multicenter, Phase II Trial of Lenvatinib for the Treatment of Patients With Anaplastic Thyroid Cancer. J Clin Oncol 2021;39:2359-66. [Crossref] [PubMed]
- Wirth LJ, Sherman E, Robinson B, et al. Efficacy of Selpercatinib in RET-Altered Thyroid Cancers. N Engl J Med 2020;383:825-35. [Crossref] [PubMed]
- Subbiah V, Hu MI, Wirth LJ, et al. Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): a multi-cohort, open-label, registrational, phase 1/2 study. Lancet Diabetes Endocrinol 2021;9:491-501. [Crossref] [PubMed]
- Hong DS, DuBois SG, Kummar S, et al. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol 2020;21:531-40. [Crossref] [PubMed]
- Doebele RC, Drilon A, Paz-Ares L, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol 2020;21:271-82. [Crossref] [PubMed]
- Dierks C, Seufert J, Aumann K, et al. Combination of Lenvatinib and Pembrolizumab Is an Effective Treatment Option for Anaplastic and Poorly Differentiated Thyroid Carcinoma. Thyroid 2021;31:1076-85. [Crossref] [PubMed]
- Iyer PC, Dadu R, Gule-Monroe M, et al. Salvage pembrolizumab added to kinase inhibitor therapy for the treatment of anaplastic thyroid carcinoma. J Immunother Cancer 2018;6:68. [Crossref] [PubMed]
- Capdevila J, Wirth LJ, Ernst T, et al. PD-1 Blockade in Anaplastic Thyroid Carcinoma. J Clin Oncol 2020;38:2620-7. [Crossref] [PubMed]
- Cabanillas ME, Dadu R, Iyer P, et al. Acquired Secondary RAS Mutation in BRAFV600E-Mutated Thyroid Cancer Patients Treated with BRAF Inhibitors. Thyroid 2020;30:1288-96. [Crossref] [PubMed]
- Nakazawa MA, Tamada Y, Tanaka Y, et al. Novel cancer subtyping method based on patient-specific gene regulatory network. Sci Rep 2021;11:23653. [Crossref] [PubMed]
- Falcone R, Conte F, Fiscon G, et al. BRAFV600E-mutant cancers display a variety of networks by SWIM analysis: prediction of vemurafenib clinical response. Endocrine 2019;64:406-13. [Crossref] [PubMed]
- Maniakas A, Dadu R, Busaidy NL, et al. Evaluation of Overall Survival in Patients With Anaplastic Thyroid Carcinoma, 2000-2019. JAMA Oncol 2020;6:1397-404. [Crossref] [PubMed]
- Cabanillas ME, Williams MD, Gunn GB, et al. Facilitating anaplastic thyroid cancer specialized treatment: A model for improving access to multidisciplinary care for patients with anaplastic thyroid cancer. Head Neck 2017;39:1291-5. [Crossref] [PubMed]


